Sensorimotor control of breathing in the mdx mouse model of Duchenne muscular dystrophy
- PMID: 28952155
- PMCID: PMC5663834
- DOI: 10.1113/JP274792
Sensorimotor control of breathing in the mdx mouse model of Duchenne muscular dystrophy
Erratum in
-
Corrigendum.J Physiol. 2018 Jan 15;596(2):343-344. doi: 10.1113/JP275606. J Physiol. 2018. PMID: 29333654 Free PMC article. No abstract available.
Abstract
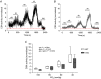
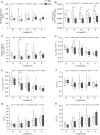
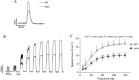
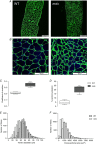
Key points: Respiratory failure is a leading cause of mortality in Duchenne muscular dystrophy (DMD), but little is known about the control of breathing in DMD and animal models. We show that young (8 weeks of age) mdx mice hypoventilate during basal breathing due to reduced tidal volume. Basal CO2 production is equivalent in wild-type and mdx mice. We show that carotid bodies from mdx mice have blunted responses to hyperoxia, revealing hypoactivity in normoxia. However, carotid body, ventilatory and metabolic responses to hypoxia are equivalent in wild-type and mdx mice. Our study revealed profound muscle weakness and muscle fibre remodelling in young mdx diaphragm, suggesting severe mechanical disadvantage in mdx mice at an early age. Our novel finding of potentiated neural motor drive to breathe in mdx mice during maximal chemoactivation suggests compensatory neuroplasticity enhancing respiratory motor output to the diaphragm and probably other accessory muscles.
Abstract: Patients with Duchenne muscular dystrophy (DMD) hypoventilate with consequential arterial blood gas derangement relevant to disease progression. Whereas deficits in DMD diaphragm are recognized, there is a paucity of knowledge in respect of the neural control of breathing in dystrophinopathies. We sought to perform an analysis of respiratory control in a model of DMD, the mdx mouse. In 8-week-old male wild-type and mdx mice, ventilation and metabolism, carotid body afferent activity, diaphragm muscle force-generating capacity, and muscle fibre size, distribution and centronucleation were determined. Diaphragm EMG activity and responsiveness to chemostimulation was determined. During normoxia, mdx mice hypoventilated, owing to a reduction in tidal volume. Basal CO2 production was not different between wild-type and mdx mice. Carotid sinus nerve responses to hyperoxia were blunted in mdx, suggesting hypoactivity. However, carotid body, ventilatory and metabolic responses to hypoxia were equivalent in wild-type and mdx mice. Diaphragm force was severely depressed in mdx mice, with evidence of fibre remodelling and damage. Diaphragm EMG responses to chemoactivation were enhanced in mdx mice. We conclude that there is evidence of chronic hypoventilation in young mdx mice. Diaphragm dysfunction confers mechanical deficiency in mdx resulting in impaired capacity to generate normal tidal volume at rest and decreased absolute ventilation during chemoactivation. Enhanced mdx diaphragm EMG responsiveness suggests compensatory neuroplasticity facilitating respiratory motor output, which may extend to accessory muscles of breathing. Our results may have relevance to emerging treatments for human DMD aiming to preserve ventilatory capacity.
Keywords: Duchenne muscular dystrophy; EMG; carotid body; diaphragm; hypoventilation; mdx.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures



References
-
- Baydur A, Gilgoff I, Prentice W, Carlson M & Fischer DA (1990). Decline in respiratory function and experience with long‐term assisted ventilation in advanced Duchenne's muscular dystrophy. Chest 97, 884–889. - PubMed
-
- Beck J, Weinberg J, Hamnegård CH, Spahija J, Olofson J, Grimby G & Sinderby C (2006). Diaphragmatic function in advanced Duchenne muscular dystrophy. Neuromuscul Disord 16, 161–167. - PubMed
-
- Bersanini C, Khirani S, Ramirez A, Lofaso F, Aubertin G, Beydon N, Mayer M, Maincent K, Boulé M & Fauroux B (2012). Nocturnal hypoxaemia and hypercapnia in children with neuromuscular disorders. Eur Respir J 39, 1206–1212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

