Iron Oxide Nanoparticles Stimulates Extra-Cellular Matrix Production in Cellular Spheroids
- PMID: 28952483
- PMCID: PMC5590449
- DOI: 10.3390/bioengineering4010004
Iron Oxide Nanoparticles Stimulates Extra-Cellular Matrix Production in Cellular Spheroids
Abstract
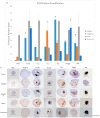
Nanotechnologies have been integrated into drug delivery, and non-invasive imaging applications, into nanostructured scaffolds for the manipulation of cells. The objective of this work was to determine how the physico-chemical properties of magnetic nanoparticles (MNPs) and their spatial distribution into cellular spheroids stimulated cells to produce an extracellular matrix (ECM). The MNP concentration (0.03 mg/mL, 0.1 mg/mL and 0.3 mg/mL), type (magnetoferritin), shape (nanorod-85 nm × 425 nm) and incorporation method were studied to determine each of their effects on the specific stimulation of four ECM proteins (collagen I, collagen IV, elastin and fibronectin) in primary rat aortic smooth muscle cell. Results demonstrated that as MNP concentration increased there was up to a 6.32-fold increase in collagen production over no MNP samples. Semi-quantitative Immunohistochemistry (IHC) results demonstrated that MNP type had the greatest influence on elastin production with a 56.28% positive area stain compared to controls and MNP shape favored elastin stimulation with a 50.19% positive area stain. Finally, there are no adverse effects of MNPs on cellular contractile ability. This study provides insight on the stimulation of ECM production in cells and tissues, which is important because it plays a critical role in regulating cellular functions.
Keywords: extracellular matrix; magnetic nanoparticles; spheroids; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Corchero J.L., Seras J., García-Fruitós E., Vazquez E., Villaverde A. Nanoparticle assisted tissue engineering. Biotechnol. Int. 2010;22:13–16.
LinkOut - more resources
Full Text Sources
Other Literature Sources

