Biotinylated N-Acetyllactosamine- and N,N-Diacetyllactosamine-Based Oligosaccharides as Novel Ligands for Human Galectin-3
- PMID: 28952509
- PMCID: PMC5590477
- DOI: 10.3390/bioengineering4020031
Biotinylated N-Acetyllactosamine- and N,N-Diacetyllactosamine-Based Oligosaccharides as Novel Ligands for Human Galectin-3
Abstract
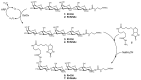
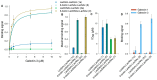
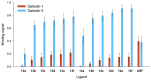
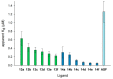
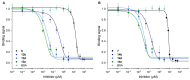
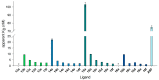
Galectin inhibitor design is an emerging research field due to the involvement of galectins in cancer. Galectin-3, in particular, plays an important role in tumor progression. To generate inhibitors, modifications of the glycan structure can be introduced. Conjugation of hydrophobic compounds to saccharides has proven to be promising as increased binding of galectin-3 can be observed. In the present study, we report on neo-glycans carrying hydrophobic biotin as novel ligands for human galectin-3. We modified N-acetyllactosamine- and N,N-diacetyllactosamine-based tetrasaccharides at the C6-position of the terminal saccharide unit using selective enzymatic oxidation and subsequent chemical conjugation of biotinamidohexanoic acid hydrazide. These neo-glycans were much better bound by galectin-3 than the unmodified counterparts. High selectivity for galectin-3 over galectin-1 was also proven. We generated multivalent neo-glycoproteins by conjugation of neo-glycans to bovine serum albumin showing high affinity for galectin-3. Compared to non-biotinylated neo-glycoproteins, we achieved high binding levels of galectin-3 with a lesser amount of conjugated neo-glycans. Multivalent ligand presentation of neo-glycoproteins significantly increased the inhibitory potency towards galectin-3 binding to asialofetuin when compared to free monovalent glycans. Our findings show the positive impact of 6-biotinylation of tetrasaccharides on galectin-3 binding, which broadens the recent design approaches for producing high-affinity ligands.
Keywords: LacdiNAc; biotin; chemo-enzymatic synthesis; galectin-3; neo-glycoprotein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Galectin Binding to Neo-Glycoproteins: LacDiNAc Conjugated BSA as Ligand for Human Galectin-3.Biomolecules. 2015 Jul 24;5(3):1671-96. doi: 10.3390/biom5031671. Biomolecules. 2015. PMID: 26213980 Free PMC article.
-
Tailored Multivalent Neo-Glycoproteins: Synthesis, Evaluation, and Application of a Library of Galectin-3-Binding Glycan Ligands.Bioconjug Chem. 2017 Nov 15;28(11):2832-2840. doi: 10.1021/acs.bioconjchem.7b00520. Epub 2017 Oct 18. Bioconjug Chem. 2017. PMID: 28976746
-
Immunoprotective neo-glycoproteins: Chemoenzymatic synthesis of multivalent glycomimetics for inhibition of cancer-related galectin-3.Eur J Med Chem. 2021 Aug 5;220:113500. doi: 10.1016/j.ejmech.2021.113500. Epub 2021 Apr 27. Eur J Med Chem. 2021. PMID: 33962190
-
Oligosaccharide specificity of galectins: a search by frontal affinity chromatography.Biochim Biophys Acta. 2002 Sep 19;1572(2-3):232-54. doi: 10.1016/s0304-4165(02)00311-2. Biochim Biophys Acta. 2002. PMID: 12223272 Review.
-
Inhibition of galectins with small molecules.Chimia (Aarau). 2011;65(1-2):18-23. doi: 10.2533/chimia.2011.18. Chimia (Aarau). 2011. PMID: 21469439 Review.
Cited by
-
Biosynthesis and Biological Significances of LacdiNAc Group on N- and O-Glycans in Human Cancer Cells.Biomolecules. 2022 Jan 24;12(2):195. doi: 10.3390/biom12020195. Biomolecules. 2022. PMID: 35204696 Free PMC article. Review.
-
Optimization of the Microwave Assisted Glycosylamines Synthesis Based on a Statistical Design of Experiments Approach.Molecules. 2020 Nov 4;25(21):5121. doi: 10.3390/molecules25215121. Molecules. 2020. PMID: 33158070 Free PMC article.
-
Functional Glyco-Nanogels for Multivalent Interaction with Lectins.Molecules. 2019 May 15;24(10):1865. doi: 10.3390/molecules24101865. Molecules. 2019. PMID: 31096570 Free PMC article.
-
Targeting Galectins With Glycomimetics.Front Chem. 2020 Aug 7;8:593. doi: 10.3389/fchem.2020.00593. eCollection 2020. Front Chem. 2020. PMID: 32850631 Free PMC article. Review.
-
Synthesis, binding affinity, and inhibitory capacity of cyclodextrin-based multivalent glycan ligands for human galectin-3.Bioorg Med Chem. 2022 Oct 15;72:116974. doi: 10.1016/j.bmc.2022.116974. Epub 2022 Sep 10. Bioorg Med Chem. 2022. PMID: 36108470 Free PMC article.
References
-
- Sasisekharan R., Myette J.R. The sweet science of glycobiology: Complex carbohydrates, molecules that are particularly important for communication among cells, are coming under systematic study. Am. Sci. 2003;91:432–441. doi: 10.1511/2003.32.888. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

