Development and Characterization of a Parallelizable Perfusion Bioreactor for 3D Cell Culture
- PMID: 28952530
- PMCID: PMC5590478
- DOI: 10.3390/bioengineering4020051
Development and Characterization of a Parallelizable Perfusion Bioreactor for 3D Cell Culture
Abstract
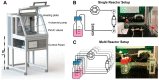
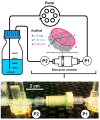
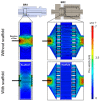
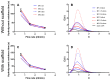
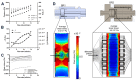
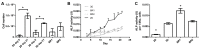
The three dimensional (3D) cultivation of stem cells in dynamic bioreactor systems is essential in the context of regenerative medicine. Still, there is a lack of bioreactor systems that allow the cultivation of multiple independent samples under different conditions while ensuring comprehensive control over the mechanical environment. Therefore, we developed a miniaturized, parallelizable perfusion bioreactor system with two different bioreactor chambers. Pressure sensors were also implemented to determine the permeability of biomaterials which allows us to approximate the shear stress conditions. To characterize the flow velocity and shear stress profile of a porous scaffold in both bioreactor chambers, a computational fluid dynamics analysis was performed. Furthermore, the mixing behavior was characterized by acquisition of the residence time distributions. Finally, the effects of the different flow and shear stress profiles of the bioreactor chambers on osteogenic differentiation of human mesenchymal stem cells were evaluated in a proof of concept study. In conclusion, the data from computational fluid dynamics and shear stress calculations were found to be predictable for relative comparison of the bioreactor geometries, but not for final determination of the optimal flow rate. However, we suggest that the system is beneficial for parallel dynamic cultivation of multiple samples for 3D cell culture processes.
Keywords: 3D cell culture; computational fluid dynamics; dynamic cultivation; fluid shear stress; perfusion bioreactor system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

