Development of Antibody-Drug Conjugates Using DDS and Molecular Imaging
- PMID: 28952557
- PMCID: PMC5615324
- DOI: 10.3390/bioengineering4030078
Development of Antibody-Drug Conjugates Using DDS and Molecular Imaging
Abstract
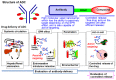
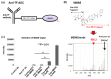
Antibody-drug conjugate (ADC), as a next generation of antibody therapeutics, is a combination of an antibody and a drug connected via a specialized linker. ADC has four action steps: systemic circulation, the enhanced permeability and retention (EPR) effect, penetration within the tumor tissue, and action on cells, such as through drug delivery system (DDS) drugs. An antibody with a size of about 10 nm has the same capacity for passive targeting as some DDS carriers, depending on the EPR effect. In addition, some antibodies are capable of active targeting. A linker is stable in the bloodstream but should release drugs efficiently in the tumor cells or their microenvironment. Thus, the linker technology is actually a typical controlled release technology in DDS. Here, we focused on molecular imaging. Fluorescent and positron emission tomography (PET) imaging is useful for the visualization and evaluation of antibody delivery in terms of passive and active targeting in the systemic circulation and in tumors. To evaluate the controlled release of the ADC in the targeted area, a mass spectrometry imaging (MSI) with a mass microscope, to visualize the drug released from ADC, was used. As a result, we succeeded in confirming the significant anti-tumor activity of anti-fibrin, or anti-tissue factor-ADC, in preclinical settings by using DDS and molecular imaging.
Keywords: ADC (antibody-drug conjugate); DDS (drug delivery system); MSI (mass spectrometry imaging); PET (positron emission tomography); antibody delivery; controlled release; molecular imaging.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

