Multicentre analysis of second-line antiretroviral treatment in HIV-infected children: adolescents at high risk of failure
- PMID: 28953325
- PMCID: PMC5640308
- DOI: 10.7448/IAS.20.1.21930
Multicentre analysis of second-line antiretroviral treatment in HIV-infected children: adolescents at high risk of failure
Abstract
Introduction: The number of HIV-infected children and adolescents requiring second-line antiretroviral treatment (ART) is increasing in low- and middle-income countries (LMIC). However, the effectiveness of paediatric second-line ART and potential risk factors for virologic failure are poorly characterized. We performed an aggregate analysis of second-line ART outcomes for children and assessed the need for paediatric third-line ART.
Methods: We performed a multicentre analysis by systematically reviewing the literature to identify cohorts of children and adolescents receiving second-line ART in LMIC, contacting the corresponding study groups and including patient-level data on virologic and clinical outcomes. Kaplan-Meier survival estimates and Cox proportional hazard models were used to describe cumulative rates and predictors of virologic failure. Virologic failure was defined as two consecutive viral load measurements >1000 copies/ml after at least six months of second-line treatment.
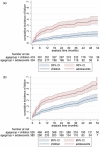
Results: We included 12 cohorts representing 928 children on second-line protease inhibitor (PI)-based ART in 14 countries in Asia and sub-Saharan Africa. After 24 months, 16.4% (95% confidence interval (CI): 13.9-19.4) of children experienced virologic failure. Adolescents (10-18 years) had failure rates of 14.5 (95% CI 11.9-17.6) per 100 person-years compared to 4.5 (95% CI 3.4-5.8) for younger children (3-9 years). Risk factors for virologic failure were adolescence (adjusted hazard ratio [aHR] 3.93, p < 0.001) and short duration of first-line ART before treatment switch (aHR 0.64 and 0.53, p = 0.008, for 24-48 months and >48 months, respectively, compared to <24 months).
Conclusions: In LMIC, paediatric PI-based second-line ART was associated with relatively low virologic failure rates. However, adolescents showed exceptionally poor virologic outcomes in LMIC, and optimizing their HIV care requires urgent attention. In addition, 16% of children and adolescents failed PI-based treatment and will require integrase inhibitors to construct salvage regimens. These drugs are currently not available in LMIC.
Keywords: HIV-1; adolescents; antiretroviral treatment; children; second-line treatment; virologic failure.
Conflict of interest statement
None to declare.
Figures
References
-
- World Health Organization Global health sector response to HIV, 2000–2015: focus on innovations in Africa: progress report [Internet]. 2015. [cited 2016 September5] Available from: http://www.who.int/hiv/pub/progressreports/2015-progress-report/en/
-
- World Health Organization Global HIV/AIDS response. Epidemic update and health sector progress towards Universal Access [Internet]. 2011. [cited 2016 September6] Available from: http://www.who.int/hiv/pub/progress_report2011/en/
-
- World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach - Second Edition 2016 [Internet]. 2016. [cited 2016 September6] Available from: http://www.who.int/hiv/pub/arv/arv-2016/en/ - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

