Natural Products as Platforms To Overcome Antibiotic Resistance
- PMID: 28953368
- PMCID: PMC5869711
- DOI: 10.1021/acs.chemrev.7b00283
Natural Products as Platforms To Overcome Antibiotic Resistance
Abstract
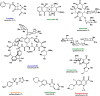
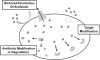
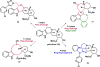
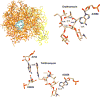
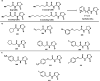
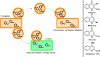
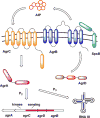
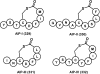
Natural products have served as powerful therapeutics against pathogenic bacteria since the golden age of antibiotics of the mid-20th century. However, the increasing frequency of antibiotic-resistant infections clearly demonstrates that new antibiotics are critical for modern medicine. Because combinatorial approaches have not yielded effective drugs, we propose that the development of new antibiotics around proven natural scaffolds is the best short-term solution to the rising crisis of antibiotic resistance. We analyze herein synthetic approaches aiming to reengineer natural products into potent antibiotics. Furthermore, we discuss approaches in modulating quorum sensing and biofilm formation as a nonlethal method, as well as narrow-spectrum pathogen-specific antibiotics, which are of interest given new insights into the implications of disrupting the microbiome.
Conflict of interest statement
The authors declare no competing financial interest.
Figures












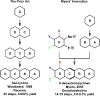
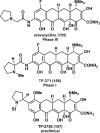

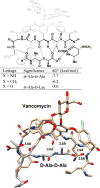

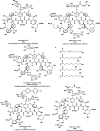










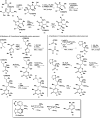


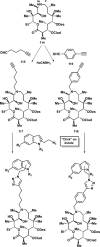
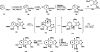
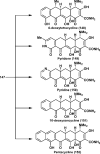
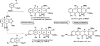



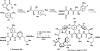
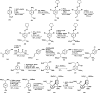


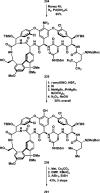





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

