Virological and immunological outcome of treatment interruption in HIV-1-infected subjects vaccinated with MVA-B
- PMID: 28953921
- PMCID: PMC5617163
- DOI: 10.1371/journal.pone.0184929
Virological and immunological outcome of treatment interruption in HIV-1-infected subjects vaccinated with MVA-B
Abstract
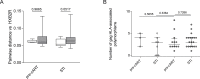
The most relevant endpoint in therapeutic HIV vaccination is the assessment of time to viral rebound or duration of sustained control of low-level viremia upon cART treatment cessation. Structured treatment interruptions (STI) are however not without risk to the patient and reliable predictors of viral rebound/control after therapeutic HIV-1 vaccination are urgently needed to ensure patient safety and guide therapeutic vaccine development. Here, we integrated immunological and virological parameters together with viral rebound dynamics after STI in a phase I therapeutic vaccine trial of a polyvalent MVA-B vaccine candidate to define predictors of viral control. Clinical parameters, proviral DNA, host HLA genetics and measures of humoral and cellular immunity were evaluated. A sieve effect analysis was conducted comparing pre-treatment viral sequences to breakthrough viruses after STI. Our results show that a reduced proviral HIV-1 DNA at study entry was independently associated with two virological parameters, delayed HIV-1 RNA rebound (p = 0.029) and lower peak viremia after treatment cessation (p = 0.019). Reduced peak viremia was also positively correlated with a decreased number of HLA class I allele associated polymorphisms in Gag sequences in the rebounding virus population (p = 0.012). Our findings suggest that proviral DNA levels and the number of HLA-associated Gag polymorphisms may have an impact on the clinical outcome of STI. Incorporation of these parameters in future therapeutic vaccine trials may guide refined immunogen design and help conduct safer STI approaches.
Conflict of interest statement
Figures



References
-
- Global AIDS Update 2016 | UNAIDS [Internet]. [cited 1 Mar 2017]. Available: http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016
-
- Crawford KW, Ripin DHB, Levin AD, Campbell JR, Flexner C, participants of Conference on Antiretroviral Drug Optimization. Optimising the manufacture, formulation, and dose of antiretroviral drugs for more cost-efficient delivery in resource-limited settings: a consensus statement. Lancet Infect Dis. 2012;12: 550–60. doi: 10.1016/S1473-3099(12)70134-2 - DOI - PubMed
-
- Holmes CB, Coggin W, Jamieson D, Mihm H, Granich R, Savio P, et al. Use of generic antiretroviral agents and cost savings in PEPFAR treatment programs. JAMA. 2010;304: 313–20. doi: 10.1001/jama.2010.993 - DOI - PubMed
-
- Katlama C, Deeks SG, Autran B, Martinez-picado J, van Lunzen J, Rouzioux C, et al. Barries to a Cure: New concepts in targeting and eradicating HIV-1 reservoirs. Lancet. 2013;381 doi: 10.1016/S0140-6736(13)60104-X.Barriers - DOI - PMC - PubMed
-
- Li JZ, Brumme ZL, Brumme CJ, Wang H, Spritzler J, Robertson MN, et al. Factors associated with viral rebound in HIV-1-infected individuals enrolled in a therapeutic HIV-1 gag vaccine trial. J Infect Dis. 2011;203: 976–83. doi: 10.1093/infdis/jiq143 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

