Chemotherapy-Associated Peripheral Neuropathy in Patients With Early-Stage Breast Cancer: A Systematic Review
- PMID: 28954296
- PMCID: PMC5825681
- DOI: 10.1093/jnci/djx140
Chemotherapy-Associated Peripheral Neuropathy in Patients With Early-Stage Breast Cancer: A Systematic Review
Abstract
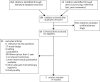
Breast cancer is the most common cancer among women worldwide, and survival rates are increasing. Chemotherapy-associated peripheral neuropathy (PN) is clinically important because of effects on quality of life (QOL) and potential effects on dose limitations. This adverse drug reaction is associated with certain classes of chemotherapy and commonly presents as peripheral sensory neuropathy whose natural course is largely unknown. The literature was reviewed to determine the frequency and characteristics of PN associated with adjuvant chemotherapy in early-stage breast cancer (ESBC) to explore the potential impact on long-term (one or more years after diagnosis) health outcomes and QOL. MEDLINE, PubMed, Embase, and the Cochrane Library were searched for relevant English-language randomized controlled trials, systematic reviews, meta-analyses, and case-control and cohort studies published between January 1990 and July 1996. Included studies were limited to current adjuvant regimens (eg, anthracyclines, taxanes, cyclophosphamide, platinum compounds). Two investigators independently reviewed abstracts, full-text articles, and extracted data from fair- and good-quality studies. Discrepancies in quality assessment and data extraction were resolved by consensus. We identified 364 articles; 60 were eligible for full-text review. Only five reports of four studies provided data beyond one year post-treatment initiation. Studies used different measures to assess PN. Neuropathic symptoms persisted in 11.0% to more than 80% of participants at one to three years following treatment. There is a paucity of data describing persistent PN in ESBC patients. Consistent use of validated measures and well-conducted randomized clinical trials or observational studies are needed to evaluate the incidence, persistence, and QOL associated with the long-term effects of PN.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Patients with breast cancer who are treated with chemotherapy risk long-term nerve damage.Cancer. 2018 Feb 15;124(4):663. doi: 10.1002/cncr.31259. Cancer. 2018. PMID: 29406588 No abstract available.
References
-
- Howlader N, Noone AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975–2013. Bethesda, MD: National Cancer Institute; 2016. http://seer.cancer.gov/csr/1975_2013/. Accessed January 9, 2017.
-
- Denduluri N, Somerfield MR, Eisen A, et al. Selection of optimal adjuvant chemotherapy regimens for human epidermal growth factor receptor 2 (HER2) – negative and adjuvant targeted therapy for HER2-positive breast cancers: An American Society of Clinical Oncology guideline adaptation of the Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2016;34(20):2416–2429. - PubMed
-
- Lee KS, Ro J, Nam BH, et al. A randomized phase-III trial of docetaxel/capecitabine vs doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008;109(3):481–489. - PubMed
-
- Toledano A, Azria D, Garaud P, et al. Phase III trial of concurrent or sequential adjuvant chemoradiotherapy after conservative surgery for early-stage breast cancer: Final results of the ARCOSEIN trial. J Clin Oncol. 2007;25(4):405–410. Erratum in: J Clin Oncol. 2007;25(16):2334. - PubMed
-
- Loprinzi CL, Thomé SD.. Understanding the utility of adjuvant systemic therapy for primary breast cancer. J Clin Oncol. 2001;19(4):972–979. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases