Heat stress induced, ligand-independent MET and EGFR signalling in hepatocellular carcinoma
- PMID: 28954551
- PMCID: PMC6175538
- DOI: 10.1080/02656736.2017.1385859
Heat stress induced, ligand-independent MET and EGFR signalling in hepatocellular carcinoma
Abstract
Purpose: The aims of the present study were 2-fold: first, to test the hypothesis that heat stress induces MET and EGFR signalling in hepatocellular carcinoma (HCC) cells and inhibition of this signalling decreases HCC clonogenic survival; and second, to identify signalling pathways associated with heat stress induced MET signalling.
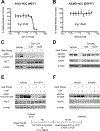
Materials and methods: MET+ and EGFR+ HCC cells were pre-treated with inhibitors to MET, EGFR, PI3K/mTOR or vehicle and subjected to heat stress or control ± HGF or EGF growth factors and assessed by colony formation assay, Western blotting and/or quantitative mass spectrometry. IACUC approved partial laser thermal or sham ablation was performed on orthotopic N1S1 and AS30D HCC tumours and liver/tumour assessed for phospho-MET and phospho-EGFR immunostaining.
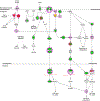
Results: Heat-stress induced rapid MET and EGFR phosphorylation that is distinct from HGF or EGF in HCC cells and thermal ablation induced MET but not EGFR phosphorylation at the HCC tumour ablation margin. Inhibition of the MET and EGFR blocked both heat stress and growth factor induced MET and EGFR phosphorylation and inhibition of MET decreased HCC clonogenic survival following heat stress. Pathway analysis of quantitative phosphoproteomic data identified downstream pathways associated with heat stress induced MET signalling including AKT, ERK, Stat3 and JNK. However, inhibition of heat stress induced MET signalling did not block AKT signalling.
Conclusions: Heat-stress induced MET and EGFR signalling is distinct from growth factor mediated signalling in HCC cells and MET inhibition enhances heat stress induced HCC cell killing via a PI3K/AKT/mTOR-independent mechanism.
Keywords: AKT; EGFR; MET; heat stress; hepatocellular carcinoma; thermal ablation.
Conflict of interest statement
Disclosure of Interest Statement
Dr. Stokes and Ms. Jia are paid employees of Cell Signaling Technology, Inc. The remaining authors report no conflicts of interest related to the contents of this manuscript. This work was supported by the National Institutes of Health under Grant C06 RR018898 and R01 CA177686.
Figures




References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. - PubMed
-
- Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210–24. Epub 2011/07/19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
