Transcriptome analysis reveals differences in mechanisms regulating cessation of luteal function in pregnant and non-pregnant dogs
- PMID: 28954628
- PMCID: PMC5618722
- DOI: 10.1186/s12864-017-4084-9
Transcriptome analysis reveals differences in mechanisms regulating cessation of luteal function in pregnant and non-pregnant dogs
Abstract
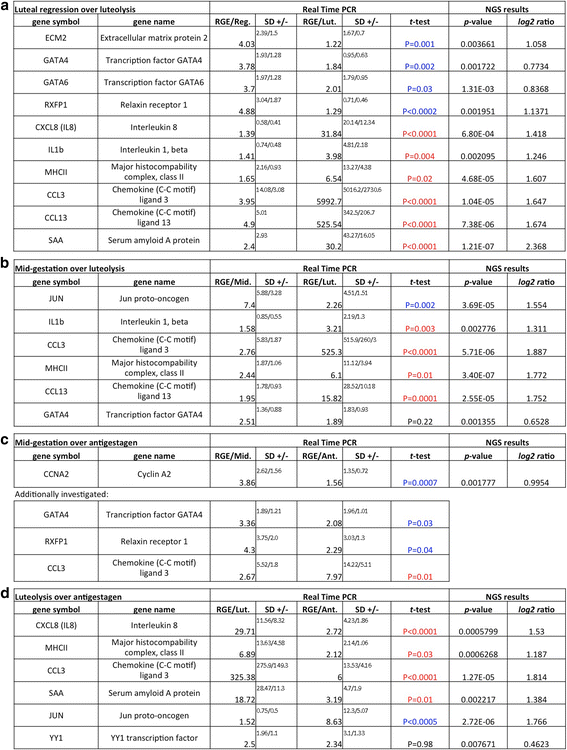
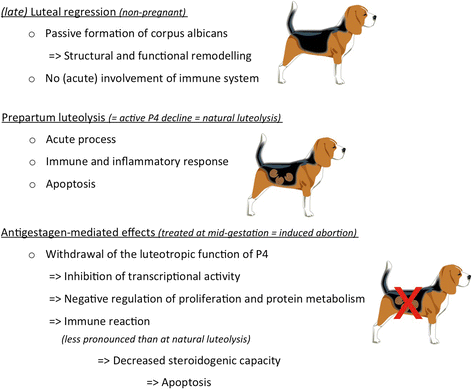
Background: In the domestic dog, corpora lutea (CL) are the only source of progesterone (P4), both in pregnant and non-pregnant cycles because there is no placental steroidogenesis. The absence of an endogenous luteolysin in absence of pregnancy results in long-lasting physiological pseudopregnancy, strongly contrasting with the acute luteolysis observed prepartum. The underlying biological mechanisms and the involvement of P4 signalling remain, however, not fully understood. Therefore, here, next-generation sequencing (RNA-Seq) was performed on CL from the late luteal phase and compared with normally luteolyzing CL collected at the prepartum P4 decrease.
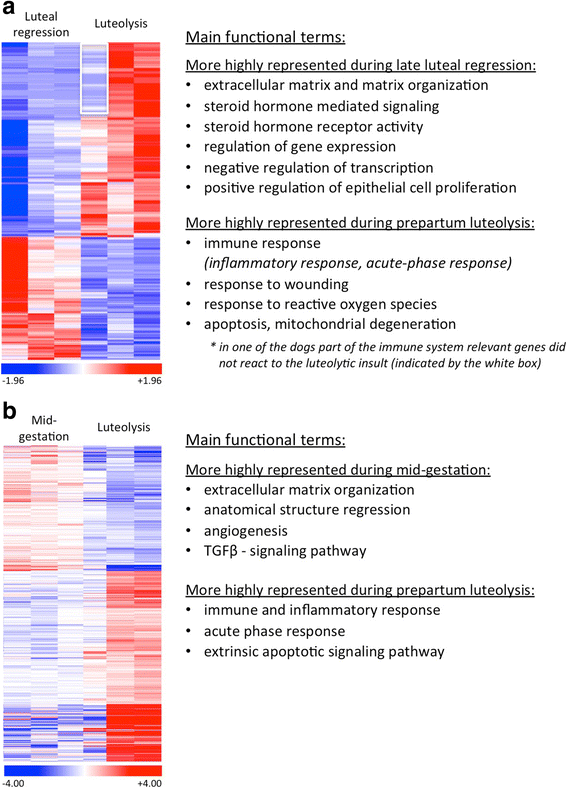
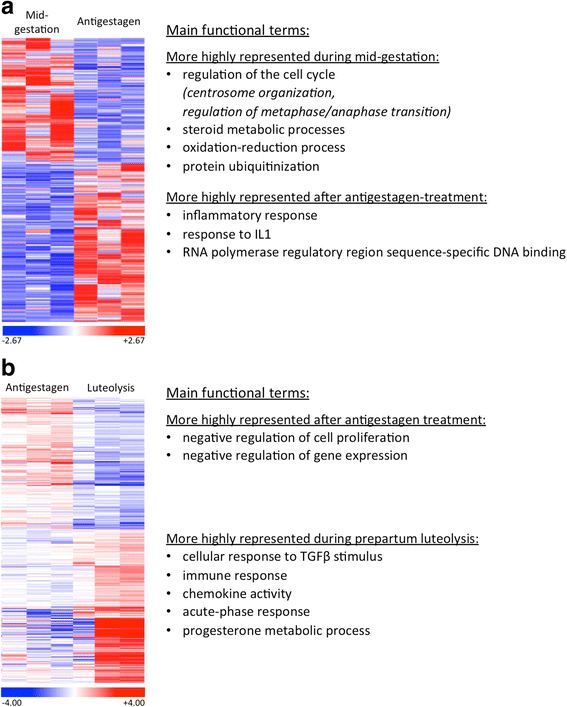
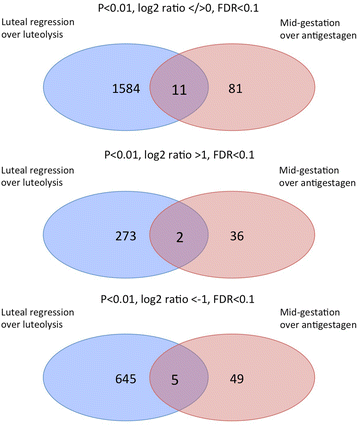
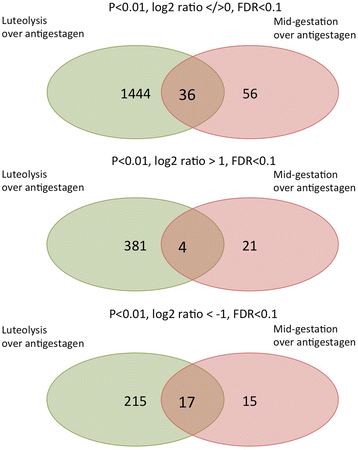
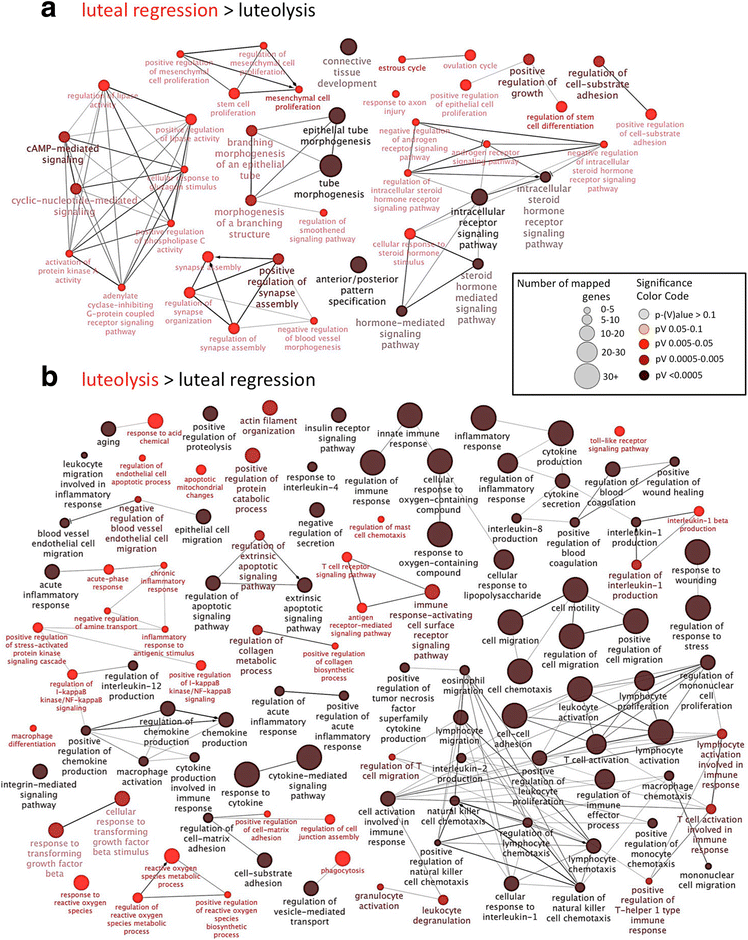
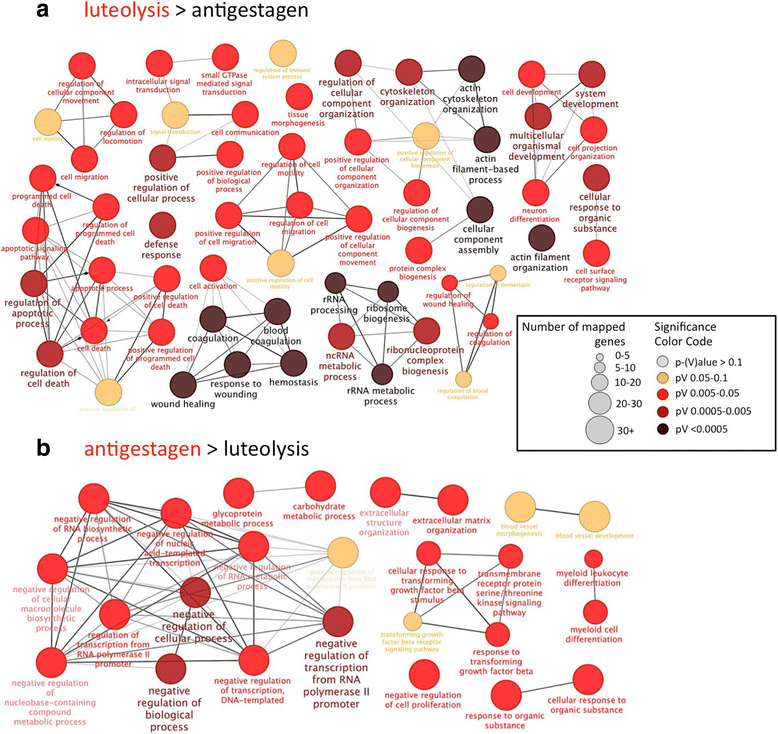
Results: The contrast "luteal regression over luteolysis" yielded 1595 differentially expressed genes (DEG). The CL in late luteal regression were predominantly associated with functional terms linked to extracellular matrix (p = 5.52e-05). Other terms related to transcriptional activity (p = 2.45e-04), and steroid hormone signalling (p = 2.29e-04), which were more highly represented in late regression than during luteolysis. The prepartum luteolysis was associated with immune inflammatory responses (p = 2.87e-14), including acute-phase reaction (p = 4.10e-06). Immune system-related events were also more highly represented in CL derived from normal luteolysis (p = 7.02e-04), compared with those from dogs in which luteolysis was induced with an antigestagen (1480 DEG in total). Additionally, the withdrawal of P4 at mid-gestation resulted in 92 DEG; over-represented terms enriched in antigestagen-treated dogs were related to the inflammatory response (p = 0.005) or response to IL1 (p = 7.29e-05). Terms related to proliferation, e.g., centrosome organization (p = 0.002) and steroid metabolic processes (p = 0.001), prevailed at mid-gestation. Thereby, our results revealed the nature of luteotropic effects of P4 within canine CL. It appears that, even though they result in diminished steroidogenic output, the effect of antigestagens is more related to the withdrawal of P4 support than to the PGF2alpha-related inflammatory reaction observed at physiological parturition.
Conclusions: We report the differential gene expression associated with maintenance and cessation of luteal function in pregnant and non-pregnant dogs. Based on the differentially expressed genes, we indicate functional pathways and gene networks that are potentially involved in the underlying endocrine and molecular mechanisms. This study establishes future research directions that may be helpful in understanding some of the clinical conditions, such as luteal insufficiency, associated with negative pregnancy outcome in dogs.
Keywords: CL; Dog (Canis familiaris); Induced abortion; Luteal regression; Prepartum luteolysis.
Conflict of interest statement
Ethics approval
Justus-Liebig University, Giessen, Germany (Regierungspräsidium Giessen, permit no. II 25.3-19c20-15c GI 18/14 and VIG3-19c-20/15c GI 18,14) and the Local Ethics Committee on Animal Experiments of the Faculty of Veterinary Medicine University of Ankara (permit no. 2006/06). Tissue materials were obtained both from clinic owned experimental animals and from privately owned dogs at owners’ consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Luteal regression vs. prepartum luteolysis: regulatory mechanisms governing canine corpus luteum function.Reprod Biol. 2014 Apr;14(2):89-102. doi: 10.1016/j.repbio.2013.11.004. Epub 2013 Dec 21. Reprod Biol. 2014. PMID: 24856467 Review.
-
Gene expression profiling of the canine placenta during normal and antigestagen-induced luteolysis.Gen Comp Endocrinol. 2019 Oct 1;282:113194. doi: 10.1016/j.ygcen.2019.05.019. Epub 2019 May 27. Gen Comp Endocrinol. 2019. PMID: 31145892
-
Cells expressing CD4, CD8, MHCII and endoglin in the canine corpus luteum of pregnancy, and prepartum activation of the luteal TNFα system.Theriogenology. 2017 Aug;98:123-132. doi: 10.1016/j.theriogenology.2017.05.003. Epub 2017 May 4. Theriogenology. 2017. PMID: 28601149
-
Luteal and placental function in the bitch: spatio-temporal changes in prolactin receptor (PRLr) expression at dioestrus, pregnancy and normal and induced parturition.Reprod Biol Endocrinol. 2011 Aug 3;9:109. doi: 10.1186/1477-7827-9-109. Reprod Biol Endocrinol. 2011. PMID: 21812980 Free PMC article.
-
Endocrine and molecular control of luteal and placental function in dogs: a review.Reprod Domest Anim. 2012 Dec;47 Suppl 6:19-24. doi: 10.1111/rda.12036. Reprod Domest Anim. 2012. PMID: 23279458 Review.
Cited by
-
Insights into the role of PGF2α in canine periparturient myometrium.Front Physiol. 2024 May 28;15:1392080. doi: 10.3389/fphys.2024.1392080. eCollection 2024. Front Physiol. 2024. PMID: 38863475 Free PMC article.
-
Transcriptome analysis reveals the molecular mechanism of hepatic metabolism disorder caused by chromium poisoning in chickens.Environ Sci Pollut Res Int. 2018 Jun;25(16):15411-15421. doi: 10.1007/s11356-018-1653-7. Epub 2018 Mar 21. Environ Sci Pollut Res Int. 2018. PMID: 29564706
-
Determination of novel reference genes for improving gene expression data normalization in selected canine reproductive tissues - a multistudy analysis.BMC Vet Res. 2020 Nov 12;16(1):440. doi: 10.1186/s12917-020-02635-6. BMC Vet Res. 2020. PMID: 33183298 Free PMC article.
-
Luteal expression of factors involved in the metabolism and sensitivity to oestrogens in the dog during pregnancy and in non-pregnant cycle.Reprod Domest Anim. 2022 Jan;57(1):86-97. doi: 10.1111/rda.14032. Epub 2021 Nov 10. Reprod Domest Anim. 2022. PMID: 34704613 Free PMC article.
-
Transcriptomic profiling of canine decidualization and effects of antigestagens on decidualized dog uterine stromal cells.Sci Rep. 2022 Dec 19;12(1):21890. doi: 10.1038/s41598-022-24790-6. Sci Rep. 2022. PMID: 36535952 Free PMC article.
References
-
- Concannon PW, McCann JP, Temple M. Biology and endocrinology of ovulation, pregnancy and parturition in the dog. J Reprod Fertil Suppl. 1989;39:3–25. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

