Receptor-mediated cell mechanosensing
- PMID: 28954860
- PMCID: PMC5687017
- DOI: 10.1091/mbc.E17-04-0228
Receptor-mediated cell mechanosensing
Abstract
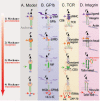
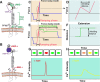
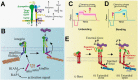
Mechanosensing describes the ability of a cell to sense mechanical cues of its microenvironment, including not only all components of force, stress, and strain but also substrate rigidity, topology, and adhesiveness. This ability is crucial for the cell to respond to the surrounding mechanical cues and adapt to the changing environment. Examples of responses and adaptation include (de)activation, proliferation/apoptosis, and (de)differentiation. Receptor-mediated cell mechanosensing is a multistep process that is initiated by binding of cell surface receptors to their ligands on the extracellular matrix or the surface of adjacent cells. Mechanical cues are presented by the ligand and received by the receptor at the binding interface; but their transmission over space and time and their conversion into biochemical signals may involve other domains and additional molecules. In this review, a four-step model is described for the receptor-mediated cell mechanosensing process. Platelet glycoprotein Ib, T-cell receptor, and integrins are used as examples to illustrate the key concepts and players in this process.
© 2017 Chen, Ju, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures



References
-
- Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

