Retromer stops beta-arrestin 1-mediated signaling from internalized cannabinoid 2 receptors
- PMID: 28954865
- PMCID: PMC5683765
- DOI: 10.1091/mbc.E17-03-0198
Retromer stops beta-arrestin 1-mediated signaling from internalized cannabinoid 2 receptors
Abstract
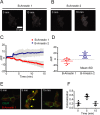
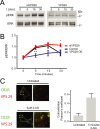
G protein-coupled receptors mediate their complex functions through activation of signaling cascades from receptors localized at the cell surface and endosomal compartments. These signaling pathways are modulated by heterotrimeric G proteins and the scaffold proteins beta-arrestin 1 and 2. However, in contrast to the events occurring at the cell surface, our knowledge of the mechanisms controlling signaling from receptors localized at intracellular compartments is still very limited. Here we sought to investigate the intracellular signaling from cannabinoid 2 receptor (CB2R). First, we show that receptor internalization is required for agonist-induced phosphorylation of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2). Then we demonstrate that ERK1/2 activation is mediated by beta-arrestin 1 from receptors localized exclusively at Rab4/5 compartments. Finally, we identify the retromer complex as a gatekeeper, terminating beta-arrestin 1-mediated ERK phosphorylation. These findings extend our understanding of the events controlling signaling from endocytosed receptors and identify the retromer as a modulator of beta-arrestin-mediated signaling from CB2R.
© 2017 Nogueras-Ortiz, Roman-Vendrell, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures





Similar articles
-
Receptor sequestration in response to β-arrestin-2 phosphorylation by ERK1/2 governs steady-state levels of GPCR cell-surface expression.Proc Natl Acad Sci U S A. 2015 Sep 15;112(37):E5160-8. doi: 10.1073/pnas.1508836112. Epub 2015 Aug 31. Proc Natl Acad Sci U S A. 2015. PMID: 26324936 Free PMC article.
-
G-protein receptor kinase 5 regulates the cannabinoid receptor 2-induced up-regulation of serotonin 2A receptors.J Biol Chem. 2013 May 31;288(22):15712-24. doi: 10.1074/jbc.M113.454843. Epub 2013 Apr 16. J Biol Chem. 2013. PMID: 23592773 Free PMC article.
-
Lack of beta-arrestin signaling in the absence of active G proteins.Nat Commun. 2018 Jan 23;9(1):341. doi: 10.1038/s41467-017-02661-3. Nat Commun. 2018. PMID: 29362459 Free PMC article.
-
Beta-arrestin signaling and regulation of transcription.J Cell Sci. 2007 Jan 15;120(Pt 2):213-8. doi: 10.1242/jcs.03338. J Cell Sci. 2007. PMID: 17215450 Review.
-
Dissecting the signaling features of the multi-protein complex GPCR/β-arrestin/ERK1/2.Eur J Cell Biol. 2018 Jun;97(5):349-358. doi: 10.1016/j.ejcb.2018.04.001. Epub 2018 Apr 4. Eur J Cell Biol. 2018. PMID: 29665971 Review.
Cited by
-
Advances in cannabinoid receptors pharmacology: from receptor structural insights to ligand discovery.Acta Pharmacol Sin. 2025 Jun;46(6):1495-1510. doi: 10.1038/s41401-024-01472-9. Epub 2025 Feb 5. Acta Pharmacol Sin. 2025. PMID: 39910211 Review.
-
Acute and long-term effects of cannabinoids on hypertension and kidney injury.Sci Rep. 2022 Apr 12;12(1):6080. doi: 10.1038/s41598-022-09902-6. Sci Rep. 2022. PMID: 35413977 Free PMC article.
-
Cannabinoid Receptor 2 (CB2) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes.ACS Pharmacol Transl Sci. 2019 Oct 1;2(6):414-428. doi: 10.1021/acsptsci.9b00049. eCollection 2019 Dec 13. ACS Pharmacol Transl Sci. 2019. PMID: 32259074 Free PMC article.
-
Cannabinoid Receptors and Ligands: Lessons from CNS Disorders and the Quest for Novel Treatment Venues.Adv Exp Med Biol. 2021;1297:43-64. doi: 10.1007/978-3-030-61663-2_4. Adv Exp Med Biol. 2021. PMID: 33537936 Free PMC article.
-
Role of Cannabinoid CB2 Receptor in Alcohol Use Disorders: From Animal to Human Studies.Int J Mol Sci. 2022 May 25;23(11):5908. doi: 10.3390/ijms23115908. Int J Mol Sci. 2022. PMID: 35682586 Free PMC article. Review.
References
-
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrié B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Eur J Biochem. 1996;237:704–711. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

