The Long 3'UTR mRNA of CaMKII Is Essential for Translation-Dependent Plasticity of Spontaneous Release in Drosophila melanogaster
- PMID: 28954869
- PMCID: PMC5666580
- DOI: 10.1523/JNEUROSCI.1313-17.2017
The Long 3'UTR mRNA of CaMKII Is Essential for Translation-Dependent Plasticity of Spontaneous Release in Drosophila melanogaster
Abstract
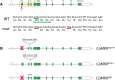
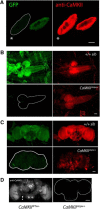
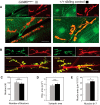
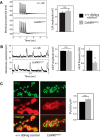
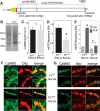
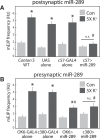
A null mutation of the Drosophila calcium/calmodulin-dependent protein kinase II gene (CaMKII) was generated using homologous recombination. Null animals survive to larval and pupal stages due to a large maternal contribution of CaMKII mRNA, which consists of a short 3'-untranslated region (UTR) form lacking regulatory elements that guide local translation. The selective loss of the long 3'UTR mRNA in CaMKII-null larvae allows us to test its role in plasticity. Development and evoked function of the larval neuromuscular junction are surprisingly normal, but the resting rate of miniature excitatory junctional potentials (mEJPs) is significantly lower in CaMKII mutants. Mutants also lack the ability to increase mEJP rate in response to spaced depolarization, a type of activity-dependent plasticity shown to require both transcription and translation. Consistent with this, overexpression of miR-289 in wild-type animals blocks plasticity of spontaneous release. In addition to the defects in regulation of mEJP rate, CaMKII protein is largely lost from synapses in the mutant. All phenotypes are non-sex-specific and rescued by a fosmid containing the entire wild-type CaMKII locus, but only viability and CaMKII localization are rescued by genomic fosmids lacking the long 3'UTR. This suggests that synaptic CaMKII accumulates by two distinct mechanisms: local synthesis requiring the long 3'UTR form of CaMKII mRNA and a process that requires zygotic transcription of CaMKII mRNA. The origin of synaptic CaMKII also dictates its functionality. Locally translated CaMKII has a privileged role in regulation of spontaneous release, which cannot be fulfilled by synaptic CaMKII from the other pool.SIGNIFICANCE STATEMENT As a regulator of synaptic development and plasticity, CaMKII has important roles in both normal and pathological function of the nervous system. CaMKII shows high conservation between Drosophila and humans, underscoring the usefulness of Drosophila in modeling its function. Drosophila CaMKII-null mutants remain viable throughout development, enabling morphological and electrophysiological characterization. Although the structure of the synapse is normal, maternally contributed CaMKII does not localize to synapses. Zygotic production of CaMKII mRNA with a long 3'-untranslated region is necessary for modulating spontaneous neurotransmission in an activity-dependent manner, but not for viability. These data argue that regulation of CaMKII localization and levels by local transcriptional processes is conserved. This is the first demonstration of distinct functions for Drosophila CaMKII mRNA variants.
Keywords: Drosophila; activity-dependent plasticity; calcium/calmodulin-dependent protein kinase II; local translation; microRNA; synaptic localization.
Copyright © 2017 the authors 0270-6474/17/3710555-13$15.00/0.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
