Sex- and Estrus-Dependent Differences in Rat Basolateral Amygdala
- PMID: 28954870
- PMCID: PMC5666581
- DOI: 10.1523/JNEUROSCI.0758-17.2017
Sex- and Estrus-Dependent Differences in Rat Basolateral Amygdala
Abstract
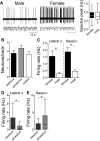
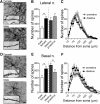
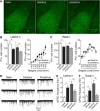
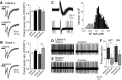
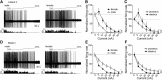
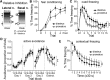
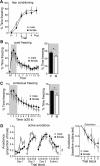
Depression and anxiety are diagnosed almost twice as often in women, and the symptomology differs in men and women and is sensitive to sex hormones. The basolateral amygdala (BLA) contributes to emotion-related behaviors that differ between males and females and across the reproductive cycle. This hints at sex- or estrus-dependent features of BLA function, about which very little is known. The purpose of this study was to test whether there are sex differences or estrous cyclicity in rat BLA physiology and to determine their mechanistic correlates. We found substantial sex differences in the activity of neurons in lateral nuclei (LAT) and basal nuclei (BA) of the BLA that were associated with greater excitatory synaptic input in females. We also found strong differences in the activity of LAT and BA neurons across the estrous cycle. These differences were associated with a shift in the inhibition-excitation balance such that LAT had relatively greater inhibition during proestrus which paralleled more rapid cued fear extinction. In contrast, BA had relatively greater inhibition during diestrus that paralleled more rapid contextual fear extinction. These results are the first to demonstrate sex differences in BLA neuronal activity and the impact of estrous cyclicity on these measures. The shift between LAT and BA predominance across the estrous cycle provides a simple construct for understanding the effects of the estrous cycle on BLA-dependent behaviors. These results provide a novel framework to understand the cyclicity of emotional memory and highlight the importance of considering ovarian cycle when studying the BLA of females.SIGNIFICANCE STATEMENT There are differences in emotional responses and many psychiatric symptoms between males and females. This may point to sex differences in limbic brain regions. Here we demonstrate sex differences in neuronal activity in one key limbic region, the basolateral amygdala (BLA), whose activity fluctuates across the estrous cycle due to a shift in the balance of inhibition and excitation across two BLA regions, the lateral and basal nuclei. By uncovering this push-pull shift between lateral and basal nuclei, these results help to explain disparate findings about the effects of biological sex and estrous cyclicity on emotion and provide a framework for understanding fluctuations in emotional memory and psychiatric symptoms.
Keywords: amygdala; electrophysiology; estrous; fear extinction; female.
Copyright © 2017 the authors 0270-6474/17/3710567-20$15.00/0.
Figures




References
-
- Aguilar R, Gil L, Gray JA, Driscoll P, Flint J, Dawson GR, Giménez-Llort L, Escorihuela RM, Fernández-Teruel A, Tobeña A (2003) Fearfulness and sex in f2 roman rats: males display more fear though both sexes share the same fearfulness traits. Physiol Behav 78:723–732. 10.1016/S0031-9384(03)00043-X - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
