Breathing above the brain stem: volitional control and attentional modulation in humans
- PMID: 28954895
- PMCID: PMC5866472
- DOI: 10.1152/jn.00551.2017
Breathing above the brain stem: volitional control and attentional modulation in humans
Abstract
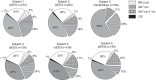
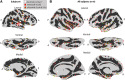
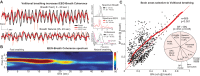
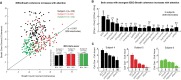
Whereas the neurophysiology of respiration has traditionally focused on automatic brain stem processes, higher brain mechanisms underlying the cognitive aspects of breathing are gaining increasing interest. Therapeutic techniques have used conscious control and awareness of breathing for millennia with little understanding of the mechanisms underlying their efficacy. Using direct intracranial recordings in humans, we correlated cortical and limbic neuronal activity as measured by the intracranial electroencephalogram (iEEG) with the breathing cycle. We show this to be the direct result of neuronal activity, as demonstrated by both the specificity of the finding to the cortical gray matter and the tracking of breath by the gamma-band (40-150 Hz) envelope in these structures. We extend prior observations by showing the iEEG signal to track the breathing cycle across a widespread network of cortical and limbic structures. We further demonstrate a sensitivity of this tracking to cognitive factors by using tasks adapted from cognitive behavioral therapy and meditative practice. Specifically, volitional control and awareness of breathing engage distinct but overlapping brain circuits. During volitionally paced breathing, iEEG-breath coherence increases in a frontotemporal-insular network, and during attention to breathing, we demonstrate increased coherence in the anterior cingulate, premotor, insular, and hippocampal cortices. Our findings suggest that breathing can act as an organizing hierarchical principle for neuronal oscillations throughout the brain and detail mechanisms of how cognitive factors impact otherwise automatic neuronal processes during interoceptive attention. NEW & NOTEWORTHY Whereas the link between breathing and brain activity has a long history of application to therapy, its neurophysiology remains unexplored. Using intracranial recordings in humans, we show neuronal activity to track the breathing cycle throughout widespread cortical/limbic sites. Volitional pacing of the breath engages frontotemporal-insular cortices, whereas attention to automatic breathing modulates the cingulate cortex. Our findings imply a fundamental role of breathing-related oscillations in driving neuronal activity and provide insight into the neuronal mechanisms of interoceptive attention.
Keywords: cortical control of breathing; interoceptive attention to breathing; intracranial EEG; mind-body; oscillations.
Figures
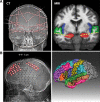
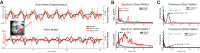
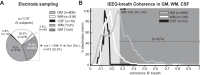
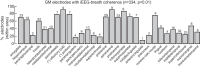

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

