Label Free Detection of L-Glutamate Using Microfluidic Based Thermal Biosensor
- PMID: 28955010
- PMCID: PMC5597124
- DOI: 10.3390/bioengineering2010002
Label Free Detection of L-Glutamate Using Microfluidic Based Thermal Biosensor
Abstract
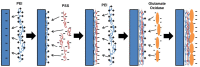
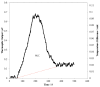
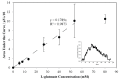
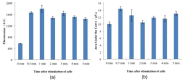
A thermoelectric biosensor for the detection of L-glutamate concentration was developed. The thermoelectric sensor is integrated into a micro-calorimeter which measures the heat produced by biochemical reactions. The device contains a single flow channel that is 120 µm high and 10 mm wide with two fluid inlets and one fluid outlet. An antimony-bismuth (Sb-Bi) thermopile with high common mode rejection ratio is attached to the lower channel wall and measures the dynamic changes in the temperature when L-glutamate undergoes oxidative deamination in the presence of glutamate oxidase (GLOD). The thermopile has a Seebeck coefficient of ~7 µV·(m·K)-1. The device geometry, together with hydrodynamic focusing, eliminates the need of extensive temperature control. Layer-by-layer assembly is used to immobilize GLOD on the surface of glass coverslips by alternate electrostatic adsorption of polyelectrolyte and GLOD. The impulse injection mode using a 6-port injection valve minimizes sample volume to 5 µL. The sensitivity of the sensor for glutamate is 17.9 nVs·mM-1 in the linear range of 0-54 mM with an R² value of 0.9873. The lowest detection limit of the sensor for glutamate is 5.3 mM.
Keywords: L-glutamate; L-glutamate oxidase; biosensor; label-free; layer-by-layer self-assembly; thermoelectric.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hamberger A., Berthold C.-H., Karlsson B., Lehmann A., Nystrom B. Extracellular GABA, glutamate and glutamine in vivo perfusion-dialysis of rabbit hippocampus. Neurol. Neurobiol. 1983;7:473–492.
LinkOut - more resources
Full Text Sources
Other Literature Sources

