Electroactive Tissue Scaffolds with Aligned Pores as Instructive Platforms for Biomimetic Tissue Engineering
- PMID: 28955011
- PMCID: PMC5597125
- DOI: 10.3390/bioengineering2010015
Electroactive Tissue Scaffolds with Aligned Pores as Instructive Platforms for Biomimetic Tissue Engineering
Abstract
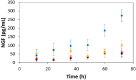
Tissues in the body are hierarchically structured composite materials with tissue-specific chemical and topographical properties. Here we report the preparation of tissue scaffolds with macroscopic pores generated via the dissolution of a sacrificial supramolecular polymer-based crystal template (urea) from a biodegradable polymer-based scaffold (polycaprolactone, PCL). Furthermore, we report a method of aligning the supramolecular polymer-based crystals within the PCL, and that the dissolution of the sacrificial urea yields scaffolds with macroscopic pores that are aligned over long, clinically-relevant distances (i.e., centimeter scale). The pores act as topographical cues to which rat Schwann cells respond by aligning with the long axis of the pores. Generation of an interpenetrating network of polypyrrole (PPy) and poly(styrene sulfonate) (PSS) in the scaffolds yields electroactive tissue scaffolds that allow the electrical stimulation of Schwann cells cultured on the scaffolds which increases the production of nerve growth factor (NGF).
Keywords: electroactive polymers; microfabrication; nerve guide; peripheral nerve; plastic electronics; topography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Instructive electroactive electrospun silk fibroin-based biomaterials for peripheral nerve tissue engineering.Biomater Adv. 2022 Oct;141:213094. doi: 10.1016/j.bioadv.2022.213094. Epub 2022 Aug 24. Biomater Adv. 2022. PMID: 36162344
-
Supracolloidal Assemblies as Sacrificial Templates for Porous Silk-Based Biomaterials.Int J Mol Sci. 2015 Aug 28;16(9):20511-22. doi: 10.3390/ijms160920511. Int J Mol Sci. 2015. PMID: 26343650 Free PMC article.
-
Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation.Acta Biomater. 2019 Sep 15;96:175-187. doi: 10.1016/j.actbio.2019.06.035. Epub 2019 Jun 29. Acta Biomater. 2019. PMID: 31260823
-
Conductive Polymeric-Based Electroactive Scaffolds for Tissue Engineering Applications: Current Progress and Challenges from Biomaterials and Manufacturing Perspectives.Int J Mol Sci. 2021 Oct 26;22(21):11543. doi: 10.3390/ijms222111543. Int J Mol Sci. 2021. PMID: 34768972 Free PMC article. Review.
-
Polypyrrole-Incorporated Conducting Constructs for Tissue Engineering Applications: A Review.Bioelectricity. 2020 Jun 1;2(2):101-119. doi: 10.1089/bioe.2020.0010. Epub 2020 Jun 17. Bioelectricity. 2020. PMID: 34471842 Free PMC article. Review.
Cited by
-
Design Strategies of Conductive Hydrogel for Biomedical Applications.Molecules. 2020 Nov 13;25(22):5296. doi: 10.3390/molecules25225296. Molecules. 2020. PMID: 33202861 Free PMC article. Review.
-
Development of an Electroactive Hydrogel as a Scaffold for Excitable Tissues.Int J Biomater. 2021 Jan 30;2021:6669504. doi: 10.1155/2021/6669504. eCollection 2021. Int J Biomater. 2021. PMID: 33603789 Free PMC article.
-
Cellulosic-Based Conductive Hydrogels for Electro-Active Tissues: A Review Summary.Gels. 2022 Feb 23;8(3):140. doi: 10.3390/gels8030140. Gels. 2022. PMID: 35323253 Free PMC article. Review.
-
Recent advances in nanotherapeutic strategies for spinal cord injury repair.Adv Drug Deliv Rev. 2019 Aug;148:38-59. doi: 10.1016/j.addr.2018.12.011. Epub 2018 Dec 22. Adv Drug Deliv Rev. 2019. PMID: 30582938 Free PMC article. Review.
-
Electrical stimulation with polypyrrole-coated polycaprolactone/silk fibroin scaffold promotes sacral nerve regeneration by modulating macrophage polarisation.Biomater Transl. 2024 Jun 28;5(2):157-174. doi: 10.12336/biomatertransl.2024.02.006. eCollection 2024. Biomater Transl. 2024. PMID: 39351163 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

