Oxidative stress and abnormal cholesterol metabolism in patients with post-cardiac arrest syndrome
- PMID: 28955127
- PMCID: PMC5612819
- DOI: 10.3164/jcbn.17-30
Oxidative stress and abnormal cholesterol metabolism in patients with post-cardiac arrest syndrome
Abstract
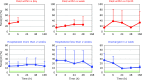
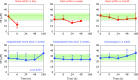
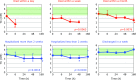
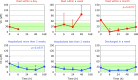
Patients with post-cardiac arrest syndrome (PCAS) suffer from whole body ischemia/reperfusion injury similar to that experienced by newborn babies. Increased oxidative stress was confirmed in PCAS patients (n = 40) at the time of hospitalization by a significant increase in the percentage of the oxidized form of coenzyme Q10 in total coenzyme Q10 compared to age-matched healthy controls (n = 55). Tissue oxidative damage in patients was suggested by the significant increase in plasma levels of free fatty acids (FFA) and the significant decrease in polyunsaturated fatty acid contents in total FFA. A greater decrease in free cholesterol (FC) compared to cholesterol esters (CE) was observed. Therefore, the FC/CE ratio significantly increased, suggesting deficiency of lecithin-cholesterol acyltransferase secreted from the liver. Time course changes of the above parameters were compared among 6 groups of patients divided according to outcome severity. Rapid declines of FC and CE were observed in patients who died within a day, while levels remained unchanged in patients discharged in a week. These data suggest that liver function is one of the key factors determining the survival of patients. Interestingly, therapeutic hypothermia treatment enhanced the increment of plasma ratio of coenzyme Q10 to total cholesterol at the end of rewarming.
Keywords: cholesterol metabolism; coenzyme Q10; plasma free fatty acids; prosaposin; therapeutic hypothermia.
Conflict of interest statement
We have not received any financial support or other benefits from commercial sources for the work reported in the manuscript. None of the authors have financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to this work.
Figures






Similar articles
-
Increased oxidative stress and renal injury in patients with sepsis.J Clin Biochem Nutr. 2018 Sep;63(2):137-143. doi: 10.3164/jcbn.17-130. Epub 2018 Mar 17. J Clin Biochem Nutr. 2018. PMID: 30279625 Free PMC article.
-
Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation.Redox Rep. 2013;18(1):12-9. doi: 10.1179/1351000212Y.0000000036. Redox Rep. 2013. PMID: 23394493 Free PMC article. Clinical Trial.
-
Increased oxidative stress and coenzyme Q10 deficiency in centenarians.J Clin Biochem Nutr. 2018 Sep;63(2):129-136. doi: 10.3164/jcbn.17-124. Epub 2018 Mar 17. J Clin Biochem Nutr. 2018. PMID: 30279624 Free PMC article.
-
Plasma marker of tissue oxidative damage and edaravone as a scavenger drug against peroxyl radicals and peroxynitrite.J Clin Biochem Nutr. 2017 Jan;60(1):49-54. doi: 10.3164/jcbn.16-63. Epub 2016 Nov 12. J Clin Biochem Nutr. 2017. PMID: 28163382 Free PMC article. Review.
-
Coagulofibrinolytic Changes in Patients with Post-cardiac Arrest Syndrome.Front Med (Lausanne). 2017 Sep 29;4:156. doi: 10.3389/fmed.2017.00156. eCollection 2017. Front Med (Lausanne). 2017. PMID: 29034235 Free PMC article. Review.
Cited by
-
Microbiome and intestinal ischemia/reperfusion injury.J Clin Biochem Nutr. 2018 Jul;63(1):26-32. doi: 10.3164/jcbn.17-137. Epub 2018 May 25. J Clin Biochem Nutr. 2018. PMID: 30087540 Free PMC article. Review.
-
Increased oxidative stress and renal injury in patients with sepsis.J Clin Biochem Nutr. 2018 Sep;63(2):137-143. doi: 10.3164/jcbn.17-130. Epub 2018 Mar 17. J Clin Biochem Nutr. 2018. PMID: 30279625 Free PMC article.
-
Simultaneous detection of reduced and oxidized forms of coenzyme Q10 in human cerebral spinal fluid as a potential marker of oxidative stress.J Clin Biochem Nutr. 2018 Nov;63(3):205-210. doi: 10.3164/jcbn.17-131. Epub 2018 Jun 8. J Clin Biochem Nutr. 2018. PMID: 30487670 Free PMC article.
-
Association between Initial Serum Cholesterol Levels and Outcomes of Patients Hospitalized after Out-of-Hospital Cardiac Arrest: A Retrospective Multicenter Registry Study.J Pers Med. 2022 Feb 7;12(2):233. doi: 10.3390/jpm12020233. J Pers Med. 2022. PMID: 35207721 Free PMC article.
-
Steroid use after cardiac arrest is associated with favourable outcomes: a systematic review and meta-analysis.J Int Med Res. 2020 May;48(5):300060520921670. doi: 10.1177/0300060520921670. J Int Med Res. 2020. PMID: 32400236 Free PMC article.
References
-
- Warner DS, Sheng H, Batinić-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–3231. - PubMed
-
- Bagheri F, Khori V, Alizadeh AM, Khalighfard S, Khodayari S, Khodayari H. Reactive oxygen species-mediated cardiac-reperfusion injury: mechanisms and therapies. Life Sci. 2016;165:43–55. - PubMed
-
- Aki HS, Fujita M, Yamashita S, et al. Elevation of jugular venous superoxide anion radical is associated with early inflammation, oxidative stress, and endothelial injury in forebrain ischemia-reperfusion rats. Brain Res. 2009;1292:180–190. - PubMed
-
- Ono T, Tsuruta R, Fujita M, et al. Xanthine oxidase is one of the major sources of superoxide anion radicals in blood after reperfusion in rats with forebrain ischemia/reperfusion. Brain Res. 2009;1305:158–167. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

