Changes in Muscle Metabolism are Associated with Phenotypic Variability in Golden Retriever Muscular Dystrophy
- PMID: 28955176
- PMCID: PMC5612180
Changes in Muscle Metabolism are Associated with Phenotypic Variability in Golden Retriever Muscular Dystrophy
Abstract
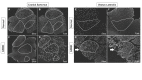
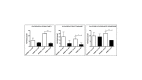
Duchenne muscular dystrophy (DMD) is an X-chromosome-linked disorder and the most common monogenic disease in people. Affected boys are diagnosed at a young age, become non-ambulatory by their early teens, and succumb to cardiorespiratory failure by their thirties. Despite being a monogenic condition resulting from mutations in the DMD gene, affected boys have noteworthy phenotypic variability. Efforts have identified genetic modifiers that could modify disease progression and be pharmacologic targets. Dogs affected with golden retriever muscular dystrophy (GRMD) have absent dystrophin and demonstrate phenotypic variability at the functional, histopathological, and molecular level. Our laboratory is particularly interested in muscle metabolism changes in dystrophin-deficient muscle. We identified several metabolic alterations, including myofiber type switching from fast (type II) to slow (type I), reduced glycolytic enzyme expression, reduced and morphologically abnormal mitochondria, and differential AMP-kinase phosphorylation (activation) between hypertrophied and wasted muscle. We hypothesize that muscle metabolism changes are, in part, responsible for phenotypic variability in GRMD. Pharmacological therapies aimed at modulating muscle metabolism can be tested in GRMD dogs for efficacy.
Keywords: AMPK; Duchenne; dystrophin; golden retriever muscular dystrophy; metabolism; muscle; phenotype.
Figures





References
-
- Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2(1):90–95. - PubMed
-
- Aartsma-Rus A, Van Deutekom JC, Fokkema IF, Van Ommen GJ, Den Dunnen JT. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34(2):135–144. - PubMed
-
- Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687-95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
