Nicotinic Acetylcholine Receptor α9 and α10 Subunits Are Expressed in the Brain of Mice
- PMID: 28955208
- PMCID: PMC5601054
- DOI: 10.3389/fncel.2017.00282
Nicotinic Acetylcholine Receptor α9 and α10 Subunits Are Expressed in the Brain of Mice
Abstract
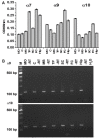
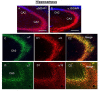
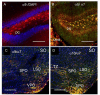
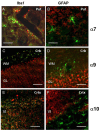
The α9 and α10 nicotinic acetylcholine receptor (nAChR) subunits are likely to be the evolutionary precursors to the entire cys-loop superfamily of ligand-gated ion channels, which includes acetylcholine, GABA, glycine and serotonin ionotropic receptors. nAChRs containing α9 and α10 subunits are found in the inner ear, dorsal root ganglia and many non-excitable tissues, but their expression in the central nervous system has not been definitely demonstrated. Here we show the presence of both α9 and α10 nAChR subunits in the mouse brain by RT-PCR and immunochemical approaches with a range of nAChR subunit-selective antibodies, which selectivity was demonstrated in the brain preparations of α7-/-, α9-/- and α10-/- mice. The α9 and α10 RNA transcripts were found in medulla oblongata (MO), cerebellum, midbrain (MB), thalamus and putamen (TP), somatosensory cortex (SC), frontal cortex (FC) and hippocampus. High α9-selective signal in ELISA was observed in the FC, SC, MO, TP and hippocampus and α10-selective signal was the highest in MO and FC. The α9 and α10 proteins were found in the brain mitochondria, while their presence on the plasma membrane has not been definitely confirmed The α7-, α9- and α10-selective antibodies stained mainly neurons and hypertrophied astrocytes, but not microglia. The α9- and α10-positive cells formed ordered structures or zones in cerebellum and superior olive (SO) and were randomly distributed among α7-positive cells in the FC; they were found in CA1, CA3 and CA4, but not in CA2 region of the hippocampus. The α9 and α10 subunits were up-regulated in α7-/- mice and both α7 and α9 subunits were down-regulated in α10-/- mice. We conclude that α9 and α10 nAChR subunits are expressed in distinct neurons of the mouse brain and in the brain mitochondria and are compensatory up-regulated in the absence of α7 subunits.
Keywords: RT-PCR; brain; immunohistochemistry; sandwich ELISA; α10 nicotinic acetylcholine receptors; α7; α9.
Figures




Comment in
-
Commentary: Nicotinic Acetylcholine Receptor α9 and α10 Subunits Are Expressed in the Brain of Mice.Front Cell Neurosci. 2018 May 1;12:104. doi: 10.3389/fncel.2018.00104. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29765305 Free PMC article. No abstract available.
Similar articles
-
Canonical and Novel Non-Canonical Cholinergic Agonists Inhibit ATP-Induced Release of Monocytic Interleukin-1β via Different Combinations of Nicotinic Acetylcholine Receptor Subunits α7, α9 and α10.Front Cell Neurosci. 2017 Jul 5;11:189. doi: 10.3389/fncel.2017.00189. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28725182 Free PMC article.
-
Acetylcholine receptors in the retinas of the α7 nicotinic acetylcholine receptor knockout mouse.Mol Vis. 2014 Sep 20;20:1328-56. eCollection 2014. Mol Vis. 2014. PMID: 25352741 Free PMC article.
-
Differential involvement of α4β2, α7 and α9α10 nicotinic acetylcholine receptors in B lymphocyte activation in vitro.Int J Biochem Cell Biol. 2011 Apr;43(4):516-24. doi: 10.1016/j.biocel.2010.12.003. Epub 2010 Dec 10. Int J Biochem Cell Biol. 2011. PMID: 21146628
-
The α9α10 acetylcholine receptor: A non-neuronal nicotinic receptor.Pharmacol Res. 2023 Apr;190:106735. doi: 10.1016/j.phrs.2023.106735. Epub 2023 Mar 15. Pharmacol Res. 2023. PMID: 36931539 Review.
-
Nicotinic acetylcholine receptors: Therapeutic targets for novel ligands to treat pain and inflammation.Pharmacol Res. 2023 Apr;190:106715. doi: 10.1016/j.phrs.2023.106715. Epub 2023 Mar 1. Pharmacol Res. 2023. PMID: 36868367 Free PMC article. Review.
Cited by
-
A Historical Review of Brain Drug Delivery.Pharmaceutics. 2022 Jun 16;14(6):1283. doi: 10.3390/pharmaceutics14061283. Pharmaceutics. 2022. PMID: 35745855 Free PMC article. Review.
-
Microglial cell response in α7 nicotinic acetylcholine receptor-deficient mice after systemic infection with Escherichia coli.J Neuroinflammation. 2022 Apr 12;19(1):94. doi: 10.1186/s12974-022-02452-8. J Neuroinflammation. 2022. PMID: 35413868 Free PMC article.
-
Mitochondria as a possible target for nicotine action.J Bioenerg Biomembr. 2019 Aug;51(4):259-276. doi: 10.1007/s10863-019-09800-z. Epub 2019 Jun 13. J Bioenerg Biomembr. 2019. PMID: 31197632 Free PMC article. Review.
-
Characterization of HA-tagged α9 and α10 nAChRs in the mouse cochlea.Sci Rep. 2020 Dec 11;10(1):21814. doi: 10.1038/s41598-020-78380-5. Sci Rep. 2020. PMID: 33311584 Free PMC article.
-
Nicotinic acetylcholine receptors regulate clustering, fusion and acidification of the rat brain synaptic vesicles.Neurochem Int. 2020 Sep;138:104779. doi: 10.1016/j.neuint.2020.104779. Epub 2020 May 29. Neurochem Int. 2020. PMID: 32474177 Free PMC article.
References
-
- Ahissar E., Staiger J. (2010). S1 laminar specialization. Scholarpedia 5:7457 Available online at: http://www.scholarpedia.org/article/S1_laminar_specialization. 10.4249/scholarpedia.7457 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

