Alterations in the Interplay between Neurons, Astrocytes and Microglia in the Rat Dentate Gyrus in Experimental Models of Neurodegeneration
- PMID: 28955220
- PMCID: PMC5601988
- DOI: 10.3389/fnagi.2017.00296
Alterations in the Interplay between Neurons, Astrocytes and Microglia in the Rat Dentate Gyrus in Experimental Models of Neurodegeneration
Abstract
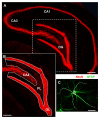
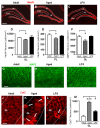
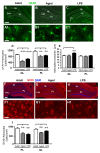
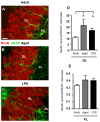
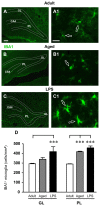
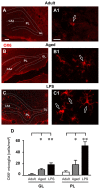
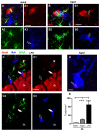
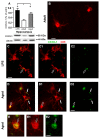
The hippocampus is negatively affected by aging and neurodegenerative diseases leading to impaired learning and memory abilities. A diverse series of progressive modifications in the intercellular communication among neurons, astrocytes and microglia occur in the hippocampus during aging or inflammation. A detailed understanding of the neurobiological modifications that contribute to hippocampal dysfunction may reveal new targets for therapeutic intervention. The current study focussed on the interplay between neurons and astroglia in the Granule Layer (GL) and the Polymorphic Layer (PL) of the Dentate Gyrus (DG) of adult, aged and LPS-treated rats. In GL and PL of aged and LPS-treated rats, astrocytes were less numerous than in adult rats. In GL of LPS-treated rats, astrocytes acquired morphological features of reactive astrocytes, such as longer branches than was observed in adult rats. Total and activated microglia increased in the aged and LPS-treated rats, as compared to adult rats. In the GL of aged and LPS-treated rats many neurons were apoptotic. Neurons decreased significantly in GL and PL of aged but not in rats treated with LPS. In PL of aged and LPS-treated rats many damaged neurons were embraced by microglia cells and were infiltrated by branches of astrocyte, which appeared to be bisecting the cell body, forming triads. Reactive microglia had a scavenging activity of dying neurons, as shown by the presence of neuronal debris within their cytoplasm. The levels of the chemokine fractalkine (CX3CL1) increased in hippocampal homogenates of aged rats and rats treated with LPS, and CX3CL1 immunoreactivity colocalized with activated microglia cells. Here we demonstrated that in the DG of aged and LPS-treated rats, astrocytes and microglia cooperate and participate in phagocytosis/phagoptosis of apoptotic granular neurons. The differential expression/activation of astroglia and the alteration of their intercommunication may be responsible for the different susceptibility of the DG in comparison to the CA1 and CA3 hippocampal areas to neurodegeneration during aging and inflammation.
Keywords: CX3CL1; MAP2; S100; apoptosis; confocal microscopy; inflammaging; phagocytosis; phagoptosis.
Figures
Similar articles
-
The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus.PLoS One. 2012;7(9):e45250. doi: 10.1371/journal.pone.0045250. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028880 Free PMC article.
-
The neuron-astrocyte-microglia triad involvement in neuroinflammaging mechanisms in the CA3 hippocampus of memory-impaired aged rats.Exp Gerontol. 2016 Oct;83:71-88. doi: 10.1016/j.exger.2016.07.011. Epub 2016 Jul 25. Exp Gerontol. 2016. PMID: 27466072
-
An Overview on the Differential Interplay Among Neurons-Astrocytes-Microglia in CA1 and CA3 Hippocampus in Hypoxia/Ischemia.Front Cell Neurosci. 2020 Nov 11;14:585833. doi: 10.3389/fncel.2020.585833. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33262692 Free PMC article. Review.
-
The neuron-astrocyte-microglia triad in CA3 after chronic cerebral hypoperfusion in the rat: Protective effect of dipyridamole.Exp Gerontol. 2017 Oct 1;96:46-62. doi: 10.1016/j.exger.2017.06.006. Epub 2017 Jun 9. Exp Gerontol. 2017. PMID: 28606482
-
The Emerging Role of the Interplay Among Astrocytes, Microglia, and Neurons in the Hippocampus in Health and Disease.Front Aging Neurosci. 2021 Apr 6;13:651973. doi: 10.3389/fnagi.2021.651973. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33889084 Free PMC article. Review.
Cited by
-
The distinct roles of calcium in rapid control of neuronal glycolysis and the tricarboxylic acid cycle.Elife. 2021 Feb 8;10:e64821. doi: 10.7554/eLife.64821. Elife. 2021. PMID: 33555254 Free PMC article.
-
A Model-Based Approach to Neuronal Electrical Activity and Spatial Organization Through the Neuronal Actin Cytoskeleton.Methods Protoc. 2025 Jul 7;8(4):76. doi: 10.3390/mps8040076. Methods Protoc. 2025. PMID: 40700314 Free PMC article.
-
Targeting Infectious Agents as a Therapeutic Strategy in Alzheimer's Disease.CNS Drugs. 2020 Jul;34(7):673-695. doi: 10.1007/s40263-020-00737-1. CNS Drugs. 2020. PMID: 32458360 Free PMC article.
-
Antioxidant thioether core-crosslinked nanoparticles prevent the bilateral spread of secondary injury to protect spatial learning and memory in a controlled cortical impact mouse model of traumatic brain injury.Biomaterials. 2021 May;272:120766. doi: 10.1016/j.biomaterials.2021.120766. Epub 2021 Mar 22. Biomaterials. 2021. PMID: 33819812 Free PMC article.
-
Butanol Extract of Tinospora cordifolia Ameliorates Cognitive Deficits Associated with Glutamate-Induced Excitotoxicity: A Mechanistic Study Using Hippocampal Neurons.Neuromolecular Med. 2020 Mar;22(1):81-99. doi: 10.1007/s12017-019-08566-2. Epub 2019 Oct 12. Neuromolecular Med. 2020. PMID: 31606849
References
-
- Amaral D. G., Lavenex P. (2007). “Hippocampal neuroanatomy,” in The Hippocampus Book, eds Andersen P., Morris R., Amaral D. G., O’Keefe J. (New York, NY: Oxford University Press; ), 37–114.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

