Assessing Metabolism and Injury in Acute Human Traumatic Brain Injury with Magnetic Resonance Spectroscopy: Current and Future Applications
- PMID: 28955291
- PMCID: PMC5600917
- DOI: 10.3389/fneur.2017.00426
Assessing Metabolism and Injury in Acute Human Traumatic Brain Injury with Magnetic Resonance Spectroscopy: Current and Future Applications
Erratum in
-
Corrigendum: Assessing Metabolism and Injury in Acute Human Traumatic Brain Injury with Magnetic Resonance Spectroscopy: Current and Future Applications.Front Neurol. 2017 Dec 1;8:642. doi: 10.3389/fneur.2017.00642. eCollection 2017. Front Neurol. 2017. PMID: 29218027 Free PMC article.
Abstract
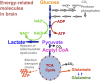
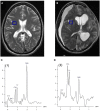
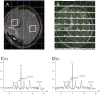
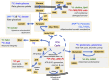
Traumatic brain injury (TBI) triggers a series of complex pathophysiological processes. These include abnormalities in brain energy metabolism; consequent to reduced tissue pO2 arising from ischemia or abnormal tissue oxygen diffusion, or due to a failure of mitochondrial function. In vivo magnetic resonance spectroscopy (MRS) allows non-invasive interrogation of brain tissue metabolism in patients with acute brain injury. Nuclei with "spin," e.g., 1H, 31P, and 13C, are detectable using MRS and are found in metabolites at various stages of energy metabolism, possessing unique signatures due to their chemical shift or spin-spin interactions (J-coupling). The most commonly used clinical MRS technique, 1H MRS, uses the great abundance of hydrogen atoms within molecules in brain tissue. Spectra acquired with longer echo-times include N-acetylaspartate (NAA), creatine, and choline. NAA, a marker of neuronal mitochondrial activity related to adenosine triphosphate (ATP), is reported to be lower in patients with TBI than healthy controls, and the ratio of NAA/creatine at early time points may correlate with clinical outcome. 1H MRS acquired with shorter echo times produces a more complex spectrum, allowing detection of a wider range of metabolites.31 P MRS detects high-energy phosphate species, which are the end products of cellular respiration: ATP and phosphocreatine (PCr). ATP is the principal form of chemical energy in living organisms, and PCr is regarded as a readily mobilized reserve for its replenishment during periods of high utilization. The ratios of high-energy phosphates are thought to represent a balance between energy generation, reserve and use in the brain. In addition, the chemical shift difference between inorganic phosphate and PCr enables calculation of intracellular pH.13 C MRS detects the 13C isotope of carbon in brain metabolites. As the natural abundance of 13C is low (1.1%), 13C MRS is typically performed following administration of 13C-enriched substrates, which permits tracking of the metabolic fate of the infused 13C in the brain over time, and calculation of metabolic rates in a range of biochemical pathways, including glycolysis, the tricarboxylic acid cycle, and glutamate-glutamine cycling. The advent of new hyperpolarization techniques to transiently boost signal in 13C-enriched MRS in vivo studies shows promise in this field, and further developments are expected.
Keywords: 13C MRS; 1H MRS; 31P MRS; biomarker; energy metabolism; trauma; traumatic brain injury.
Figures

Similar articles
-
Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate.Psychiatry Res. 2003 Jun 30;123(2):87-100. doi: 10.1016/s0925-4927(03)00046-5. Psychiatry Res. 2003. PMID: 12850248
-
Cerebral metabolism in experimental hydrocephalus: an in vivo 1H and 31P magnetic resonance spectroscopy study.J Neurosurg. 1999 Oct;91(4):660-8. doi: 10.3171/jns.1999.91.4.0660. J Neurosurg. 1999. PMID: 10507389
-
Hyperpolarized α-keto[1-13C]isocaproate as a 13C magnetic resonance spectroscopic agent for profiling branched chain amino acid metabolism in tumors.2010 Jan 15 [updated 2010 May 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jan 15 [updated 2010 May 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641995 Free Books & Documents. Review.
-
Magnetic resonance spectroscopy in traumatic brain injury.J Head Trauma Rehabil. 2001 Apr;16(2):149-64. doi: 10.1097/00001199-200104000-00005. J Head Trauma Rehabil. 2001. PMID: 11275576 Review.
-
Hyperpolarized 13C-labeled bicarbonate (H13CO3-) for in vivo pH measurement with 13C magnetic resonance spectroscopy.2010 Jan 25 [updated 2010 Apr 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jan 25 [updated 2010 Apr 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641986 Free Books & Documents. Review.
Cited by
-
Spatio-temporal metabolic rewiring in the brain of TgF344-AD rat model of Alzheimer's disease.Sci Rep. 2022 Oct 10;12(1):16958. doi: 10.1038/s41598-022-20962-6. Sci Rep. 2022. PMID: 36216838 Free PMC article.
-
Dysregulated Glucose Metabolism as a Therapeutic Target to Reduce Post-traumatic Epilepsy.Front Cell Neurosci. 2018 Oct 16;12:350. doi: 10.3389/fncel.2018.00350. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30459556 Free PMC article. Review.
-
Pyruvate Dehydrogenase and Tricarboxylic Acid Cycle Enzymes Are Sensitive Targets of Traumatic Brain Injury Induced Metabolic Derangement.Int J Mol Sci. 2019 Nov 16;20(22):5774. doi: 10.3390/ijms20225774. Int J Mol Sci. 2019. PMID: 31744143 Free PMC article.
-
Serum metabolome associated with severity of acute traumatic brain injury.Nat Commun. 2022 May 10;13(1):2545. doi: 10.1038/s41467-022-30227-5. Nat Commun. 2022. PMID: 35538079 Free PMC article.
-
Crosstalk Between the Gut Microbiota and the Brain: An Update on Neuroimaging Findings.Front Neurol. 2019 Aug 13;10:883. doi: 10.3389/fneur.2019.00883. eCollection 2019. Front Neurol. 2019. PMID: 31456743 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

