Co-delivery of Dual Toll-Like Receptor Agonists and Antigen in Poly(Lactic-Co-Glycolic) Acid/Polyethylenimine Cationic Hybrid Nanoparticles Promote Efficient In Vivo Immune Responses
- PMID: 28955328
- PMCID: PMC5601407
- DOI: 10.3389/fimmu.2017.01077
Co-delivery of Dual Toll-Like Receptor Agonists and Antigen in Poly(Lactic-Co-Glycolic) Acid/Polyethylenimine Cationic Hybrid Nanoparticles Promote Efficient In Vivo Immune Responses
Abstract
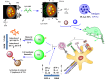
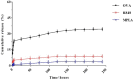
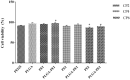
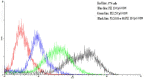
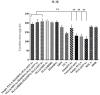
Strategies to design delivery vehicles are critical in modern vaccine-adjuvant development. Nanoparticles (NPs) encapsulating antigen(s) and adjuvant(s) are promising vehicles to deliver antigen(s) and adjuvant(s) to antigen-presenting cells (APCs), allowing optimal immune responses against a specific pathogen. In this study, we developed a novel adjuvant delivery approach for induction of efficient in vivo immune responses. Polyethylenimine (PEI) was physically conjugated to poly(lactic-co-glycolic) acid (PLGA) to form PLGA/PEI NPs. This complex was encapsulated with resiquimod (R848) as toll-like receptor (TLR) 7/8 agonist, or monophosphoryl lipid A (MPLA) as TLR4 agonist and co-assembled with cytosine-phosphorothioate-guanine oligodeoxynucleotide (CpG ODN) as TLR9 agonist to form a tripartite formulation [two TLR agonists (inside and outside NPs) and PLGA/PEI NPs as delivery system]. The physicochemical characteristics, cytotoxicity and cellular uptake of these synthesized delivery vehicles were investigated. Cellular viability test revealed no pronounced cytotoxicity as well as increased cellular uptake compared to control groups in murine macrophage cells (J774 cell line). In the next step, PLGA (MPLA or R848)/PEI (CpG ODN) were co-delivered with ovalbumin (OVA) encapsulated into PLGA NPs to enhance the induction of immune responses. The immunogenicity properties of these co-delivery formulations were examined in vivo by evaluating the cytokine (IFN-γ, IL-4, and IL-1β) secretion and antibody (IgG1, IgG2a) production. Robust and efficient immune responses were achieved after in vivo administration of PLGA (MPLA or R848)/PEI (CpG ODN) co-delivered with OVA encapsulated in PLGA NPs in BALB/c mice. Our results demonstrate a rational design of using dual TLR agonists in a context-dependent manner for efficient nanoparticulate adjuvant-vaccine development.
Keywords: CpG ODN; adjuvants; monophosphoryl lipid A; poly(lactic-co-glycolic) acid nanoparticles; polyethylenimine; resiquimod; toll-like receptor agonist; vaccine.
Figures




Similar articles
-
Induction of a balanced Th1/Th2 immune responses by co-delivery of PLGA/ovalbumin nanospheres and CpG ODNs/PEI-SWCNT nanoparticles as TLR9 agonist in BALB/c mice.Int J Pharm. 2016 Dec 30;515(1-2):708-720. doi: 10.1016/j.ijpharm.2016.10.065. Epub 2016 Oct 29. Int J Pharm. 2016. PMID: 27989827
-
The Immunoenhancement Effects of Polyethylenimine-Modified Chinese Yam Polysaccharide-Encapsulated PLGA Nanoparticles as an Adjuvant.Int J Nanomedicine. 2020 Aug 5;15:5527-5543. doi: 10.2147/IJN.S252515. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32848386 Free PMC article.
-
Immunostimulatory Pickering emulsion for oral vaccine delivery.Int J Pharm. 2025 Aug 20;681:125890. doi: 10.1016/j.ijpharm.2025.125890. Epub 2025 Jun 23. Int J Pharm. 2025. PMID: 40562287
-
Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant.Adv Drug Deliv Rev. 2013 Oct;65(10):1386-99. doi: 10.1016/j.addr.2013.05.013. Epub 2013 Jun 7. Adv Drug Deliv Rev. 2013. PMID: 23751781 Review.
-
Safety and efficacy of toll-like receptor agonists as therapeutic agents and vaccine adjuvants for infectious diseases in animals: a systematic review.Front Vet Sci. 2024 Sep 17;11:1428713. doi: 10.3389/fvets.2024.1428713. eCollection 2024. Front Vet Sci. 2024. PMID: 39355141 Free PMC article.
Cited by
-
PEI-Engineered Lipid@PLGA Hybrid Nanoparticles for Multimodal Delivery of Antigens and Immune Adjuvants to the Respiratory Mucosa.Adv Healthc Mater. 2024 Dec;13(32):e2402688. doi: 10.1002/adhm.202402688. Epub 2024 Sep 11. Adv Healthc Mater. 2024. PMID: 39258393 Free PMC article.
-
Biomaterials as Tools to Decode Immunity.Adv Mater. 2020 Apr;32(13):e1903367. doi: 10.1002/adma.201903367. Epub 2019 Nov 29. Adv Mater. 2020. PMID: 31782844 Free PMC article. Review.
-
Cationic Nanostructures for Vaccines Design.Biomimetics (Basel). 2020 Jul 7;5(3):32. doi: 10.3390/biomimetics5030032. Biomimetics (Basel). 2020. PMID: 32645946 Free PMC article. Review.
-
Orthogonal modular biosynthesis of nanoscale conjugate vaccines for vaccination against infection.Nano Res. 2022;15(2):1645-1653. doi: 10.1007/s12274-021-3713-4. Epub 2021 Aug 12. Nano Res. 2022. PMID: 34405037 Free PMC article.
-
Monocytes as a convergent nanoparticle therapeutic target for cardiovascular diseases.Adv Drug Deliv Rev. 2022 Mar;182:114116. doi: 10.1016/j.addr.2022.114116. Epub 2022 Jan 24. Adv Drug Deliv Rev. 2022. PMID: 35085623 Free PMC article.
References
-
- Haghparast A, Zakeri A, Ebrahimian M, Ramezani M. Targeting pattern recognition receptors (PRRs) in nano-adjuvants: current perspectives. Curr Bionanotechnol (2016) 2(1):47–59.10.2174/2213529402666160601125159 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

