Host- Toxoplasma gondii Coadaptation Leads to Fine Tuning of the Immune Response
- PMID: 28955329
- PMCID: PMC5601305
- DOI: 10.3389/fimmu.2017.01080
Host- Toxoplasma gondii Coadaptation Leads to Fine Tuning of the Immune Response
Abstract
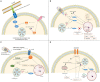
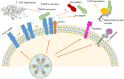
Toxoplasma gondii has successfully developed strategies to evade host's immune response and reach immune privileged sites, which remains in a controlled environment inside quiescent tissue cysts. In this review, we will approach several known mechanisms used by the parasite to modulate mainly the murine immune system at its favor. In what follows, we review recent findings revealing interference of host's cell autonomous immunity and cell signaling, gene expression, apoptosis, and production of microbicide molecules such as nitric oxide and oxygen reactive species during parasite infection. Modulation of host's metalloproteinases of extracellular matrix is also discussed. These immune evasion strategies are determinant to parasite dissemination throughout the host taking advantage of cells from the immune system to reach brain and retina, crossing crucial hosts' barriers.
Keywords: T cells; Toxoplasma gondii; cell signalling; immunity; immunomodulation.
Figures
Similar articles
-
Subversion of innate and adaptive immune responses by Toxoplasma gondii.Parasitol Res. 2007 Jan;100(2):191-203. doi: 10.1007/s00436-006-0306-9. Epub 2006 Oct 6. Parasitol Res. 2007. PMID: 17024357 Review.
-
[Immune evasion molecules of Toxoplasma gondii].Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2012 Oct 30;30(5):396-400. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2012. PMID: 23484284 Review. Chinese.
-
Overview of Apoptosis, Autophagy, and Inflammatory Processes in Toxoplasma gondii Infected Cells.Pathogens. 2023 Feb 4;12(2):253. doi: 10.3390/pathogens12020253. Pathogens. 2023. PMID: 36839525 Free PMC article. Review.
-
Phytoecdysteroids as modulators of the Toxoplasma gondii growth rate in human and mouse cells.Parasit Vectors. 2015 Aug 15;8:422. doi: 10.1186/s13071-015-1019-7. Parasit Vectors. 2015. PMID: 26272689 Free PMC article.
-
Modelling parasite dissemination: host cell subversion and immune evasion by Toxoplasma gondii.Cell Microbiol. 2010 Mar;12(3):292-300. doi: 10.1111/j.1462-5822.2009.01417.x. Epub 2009 Dec 8. Cell Microbiol. 2010. PMID: 19995386 Review.
Cited by
-
Animal venoms: a novel source of anti-Toxoplasma gondii drug candidates.Front Pharmacol. 2023 May 3;14:1178070. doi: 10.3389/fphar.2023.1178070. eCollection 2023. Front Pharmacol. 2023. PMID: 37205912 Free PMC article. Review.
-
From Entry to Early Dissemination-Toxoplasma gondii's Initial Encounter With Its Host.Front Cell Infect Microbiol. 2019 Mar 5;9:46. doi: 10.3389/fcimb.2019.00046. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30891433 Free PMC article. Review.
-
Toxoplasma gondii ROP18 inhibits human glioblastoma cell apoptosis through a mitochondrial pathway by targeting host cell P2X1.Parasit Vectors. 2019 Jun 4;12(1):284. doi: 10.1186/s13071-019-3529-1. Parasit Vectors. 2019. PMID: 31164145 Free PMC article.
-
Inhibition of Src signaling induces autophagic killing of Toxoplasma gondii via PTEN-mediated deactivation of Akt.PLoS Pathog. 2025 Jan 27;21(1):e1012907. doi: 10.1371/journal.ppat.1012907. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39869638 Free PMC article.
-
Natural phytochemicals that affect autophagy in the treatment of oral diseases and infections: A review.Front Pharmacol. 2022 Aug 25;13:970596. doi: 10.3389/fphar.2022.970596. eCollection 2022. Front Pharmacol. 2022. PMID: 36091810 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

