The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy
- PMID: 28955340
- PMCID: PMC5601256
- DOI: 10.3389/fimmu.2017.01124
The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy
Abstract
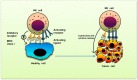
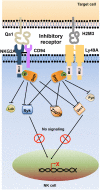
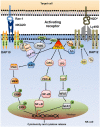
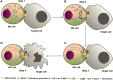
Natural killer (NK) cells are innate immune cells that show strong cytolytic function against physiologically stressed cells such as tumor cells and virus-infected cells. NK cells show a broad array of tissue distribution and phenotypic variability. NK cells express several activating and inhibitory receptors that recognize the altered expression of proteins on target cells and control the cytolytic function. NK cells have been used in several clinical trials to control tumor growth. However, the results are encouraging only in hematological malignancies but not very promising in solid tumors. Increasing evidence suggests that tumor microenvironment regulate the phenotype and function of NK cells. In this review, we discussed the NK cell phenotypes and its effector function and impact of the tumor microenvironment on effector and cytolytic function of NK cells. We also summarized various NK cell-based immunotherapeutic strategies used in the past and the possibilities to improve the function of NK cell for the better clinical outcome.
Keywords: cancer immunotherapy; inflammation; innate immune cells; natural killer cells; tumor microenvironment.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

