Mass Cytometry Identifies Distinct Lung CD4+ T Cell Patterns in Löfgren's Syndrome and Non-Löfgren's Syndrome Sarcoidosis
- PMID: 28955342
- PMCID: PMC5601005
- DOI: 10.3389/fimmu.2017.01130
Mass Cytometry Identifies Distinct Lung CD4+ T Cell Patterns in Löfgren's Syndrome and Non-Löfgren's Syndrome Sarcoidosis
Abstract
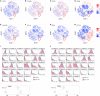
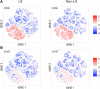
Sarcoidosis is a granulomatous disorder of unknown etiology, characterized by accumulation of activated CD4+ T cells in the lungs. Disease phenotypes Löfgren's syndrome (LS) and "non-LS" differ in terms of clinical manifestations, genetic background, HLA association, and prognosis, but the underlying inflammatory mechanisms largely remain unknown. Bronchoalveolar lavage fluid cells from four HLA-DRB1*03+ LS and four HLA-DRB1*03- non-LS patients were analyzed by mass cytometry, using a panel of 33 unique markers. Differentially regulated CD4+ T cell populations were identified using the Citrus algorithm, and t-stochastic neighborhood embedding was applied for dimensionality reduction and single-cell data visualization. We identified 19 individual CD4+ T cell clusters differing significantly in abundance between LS and non-LS patients. Seven clusters more frequent in LS patients were characterized by significantly higher expression of regulatory receptors CTLA-4, PD-1, and ICOS, along with low expression of adhesion marker CD44. In contrast, 12 clusters primarily found in non-LS displayed elevated expression of activation and effector markers HLA-DR, CD127, CD39, as well as CD44. Hierarchical clustering further indicated functional heterogeneity and diverse origins of T cell receptor Vα2.3/Vβ22-restricted cells in LS. Finally, a near-complete overlap of CD8 and Ki-67 expression suggested larger influence of CD8+ T cell activity on sarcoid inflammation than previously appreciated. In this study, we provide detailed characterization of pulmonary T cells and immunological parameters that define separate disease pathways in LS and non-LS. With direct association to clinical parameters, such as granuloma persistence, resolution, or chronic inflammation, these results provide a valuable foundation for further exploration and potential clinical application.
Keywords: CD4+ T cells; Löfgren’s syndrome; bronchoalveolar lavage; disease phenotypes; granuloma; mass cytometry; sarcoidosis.
Figures




Similar articles
-
Th17-lineage cells in pulmonary sarcoidosis and Löfgren's syndrome: Friend or foe?J Autoimmun. 2018 Feb;87:82-96. doi: 10.1016/j.jaut.2017.12.012. Epub 2018 Jan 5. J Autoimmun. 2018. PMID: 29310925 Review.
-
Bronchoalveolar lavage characteristics correlate with HLA tag SNPs in patients with Löfgren's syndrome and other sarcoidosis.Clin Exp Immunol. 2019 May;196(2):249-258. doi: 10.1111/cei.13257. Epub 2019 Jan 24. Clin Exp Immunol. 2019. PMID: 30585624 Free PMC article. Clinical Trial.
-
Reduced expression of peroxisome proliferator-activated receptor alpha in BAL and blood T cells of non-löfgren's sarcoidosis patients.J Inflamm (Lond). 2015 Apr 9;12:28. doi: 10.1186/s12950-015-0071-6. eCollection 2015. J Inflamm (Lond). 2015. PMID: 25969669 Free PMC article.
-
Lung CD4+ Vα2.3+ T-cells in sarcoidosis cohorts with Löfgren's syndrome.Respir Res. 2020 Feb 28;21(1):61. doi: 10.1186/s12931-020-1327-0. Respir Res. 2020. PMID: 32111204 Free PMC article.
-
HLA associations and Löfgren's syndrome.Expert Rev Clin Immunol. 2012 Jan;8(1):55-62. doi: 10.1586/eci.11.76. Expert Rev Clin Immunol. 2012. PMID: 22149340 Review.
Cited by
-
CD31+, CD38+, CD44+, and CD103+ lymphocytes in peripheral blood, bronchoalveolar lavage fluid and lung biopsy tissue in sarcoid patients and controls.J Thorac Dis. 2021 Apr;13(4):2300-2318. doi: 10.21037/jtd-20-2396. J Thorac Dis. 2021. PMID: 34012580 Free PMC article.
-
A fungal antigenic driver for Löfgren's syndrome sarcoidosis.J Exp Med. 2021 Oct 4;218(10):e20211572. doi: 10.1084/jem.20211572. Epub 2021 Sep 13. J Exp Med. 2021. PMID: 34515726 Free PMC article.
-
The broad spectrum of lung diseases in primary antibody deficiencies.Eur Respir Rev. 2018 Aug 29;27(149):180019. doi: 10.1183/16000617.0019-2018. Print 2018 Sep 30. Eur Respir Rev. 2018. PMID: 30158276 Free PMC article. Review.
-
From COVID-19 to Sarcoidosis: How Similar Are These Two Diseases?Front Immunol. 2022 May 9;13:877303. doi: 10.3389/fimmu.2022.877303. eCollection 2022. Front Immunol. 2022. PMID: 35615369 Free PMC article. Review.
-
Familial risk and phenotypic variation of sarcoidosis in the Icelandic population.ERJ Open Res. 2025 Jun 16;11(3):00964-2024. doi: 10.1183/23120541.00964-2024. eCollection 2025 May. ERJ Open Res. 2025. PMID: 40524927 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

