Insights into Resistance to Fe Deficiency Stress from a Comparative Study of In Vitro-Selected Novel Fe-Efficient and Fe-Inefficient Potato Plants
- PMID: 28955367
- PMCID: PMC5601415
- DOI: 10.3389/fpls.2017.01581
Insights into Resistance to Fe Deficiency Stress from a Comparative Study of In Vitro-Selected Novel Fe-Efficient and Fe-Inefficient Potato Plants
Abstract
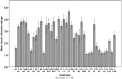
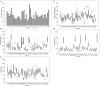
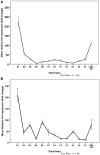
Iron (Fe) deficiency induces chlorosis (IDC) in plants and can result in reduced plant productivity. Therefore, development of Fe-efficient plants is of great interest. To gain a better understanding of the physiology of Fe-efficient plants, putative novel plant variants were regenerated from potato (Solanum tubersosum L. var. 'Iwa') callus cultures selected under Fe deficient or low Fe supply (0-5 μM Fe). Based on visual chlorosis rating (VCR), 23% of callus-derived regenerants were classified as Fe-efficient (EF) and 77% as Fe-inefficient (IFN) plant lines when they were grown under Fe deficiency conditions. Stem height was found to be highly correlated with internodal distance, leaf and root lengths in the EF plant lines grown under Fe deficiency conditions. In addition, compared to the IFN plant lines and control parental biotype, the EF plants including the lines named A1, B2, and B9, exhibited enhanced formation of lateral roots and root hairs as well as increased expression of ferritin (fer3) in the leaf and iron-regulated transporter (irt1) in the root. These morphological adaptations and changes in expression the fer3 and irt1 genes of the selected EF potato lines suggest that they are associated with resistance to low Fe supply stress.
Keywords: Fe-deficiency; chlorosis; ferritin; iron-regulated transporter; stress resistance.
Figures





Similar articles
-
Iron partitioning at an early growth stage impacts iron deficiency responses in soybean plants (Glycine max L.).Front Plant Sci. 2015 May 12;6:325. doi: 10.3389/fpls.2015.00325. eCollection 2015. Front Plant Sci. 2015. PMID: 26029227 Free PMC article.
-
High relative air humidity influences mineral accumulation and growth in iron deficient soybean plants.Front Plant Sci. 2014 Dec 18;5:726. doi: 10.3389/fpls.2014.00726. eCollection 2014. Front Plant Sci. 2014. PMID: 25566297 Free PMC article.
-
Rhizobacteria-Mediated Activation of the Fe Deficiency Response in Arabidopsis Roots: Impact on Fe Status and Signaling.Front Plant Sci. 2019 Jul 12;10:909. doi: 10.3389/fpls.2019.00909. eCollection 2019. Front Plant Sci. 2019. PMID: 31354776 Free PMC article.
-
Exogenous Melatonin Improves Plant Iron Deficiency Tolerance via Increased Accumulation of Polyamine-Mediated Nitric Oxide.Int J Mol Sci. 2016 Oct 25;17(11):1777. doi: 10.3390/ijms17111777. Int J Mol Sci. 2016. PMID: 27792144 Free PMC article.
-
The Understanding of the Plant Iron Deficiency Responses in Strategy I Plants and the Role of Ethylene in This Process by Omic Approaches.Front Plant Sci. 2017 Jan 24;8:40. doi: 10.3389/fpls.2017.00040. eCollection 2017. Front Plant Sci. 2017. PMID: 28174585 Free PMC article. Review.
Cited by
-
The activation of iron deficiency responses of grapevine rootstocks is dependent to the availability of the nitrogen forms.BMC Plant Biol. 2024 Mar 26;24(1):218. doi: 10.1186/s12870-024-04906-y. BMC Plant Biol. 2024. PMID: 38532351 Free PMC article.
References
-
- Barton L. L., Abadía J. (2006). Iron Nutrition in Plants and Rhizospheric Microorganisms. Springer.
LinkOut - more resources
Full Text Sources
Other Literature Sources

