RIG-I-Like Receptor Signaling in Singleton-Merten Syndrome
- PMID: 28955379
- PMCID: PMC5600918
- DOI: 10.3389/fgene.2017.00118
RIG-I-Like Receptor Signaling in Singleton-Merten Syndrome
Abstract
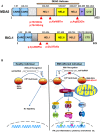
Singleton-Merten syndrome (SMS) is an autosomal dominant, multi-system innate immune disorder characterized by early and severe aortic and valvular calcification, dental and skeletal abnormalities, psoriasis, glaucoma, and other varying clinical findings. Recently we identified a specific gain-of-function mutation in IFIH1, interferon induced with helicase C domain 1, segregated with this disease. SMS disease without hallmark dental anomalies, termed atypical SMS, has recently been reported caused by variants in DDX58, DEXD/H-box helicase 58. IFIH1 and DDX58 encode retinoic acid-inducible gene I (RIG-I)-like receptors family members melanoma differentiation-associated gene 5 and RIG-I, respectively. These cytosolic pattern recognition receptors function in viral RNA detection initiating an innate immune response through independent pathways that promote type I and type III interferon expression and proinflammatory cytokines. In this review, we focus on SMS as an innate immune disorder summarizing clinical features, molecular aspects of the pathogenetic pathway and discussing underlying mechanisms of the disease.
Keywords: DDX58; IFIH1; MDA5; RIG-I; Singleton-Merten syndrome.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

