RNA-sequencing reveals the complexities of the transcriptional response to lignocellulosic biofuel substrates in Aspergillus niger
- PMID: 28955445
- PMCID: PMC5598271
- DOI: 10.1186/s40694-014-0003-x
RNA-sequencing reveals the complexities of the transcriptional response to lignocellulosic biofuel substrates in Aspergillus niger
Abstract
Background: Saprobic fungi are the predominant industrial sources of Carbohydrate Active enZymes (CAZymes) used for the saccharification of lignocellulose during the production of second generation biofuels. The production of more effective enzyme cocktails is a key objective for efficient biofuel production. To achieve this objective, it is crucial to understand the response of fungi to lignocellulose substrates. Our previous study used RNA-seq to identify the genes induced in Aspergillus niger in response to wheat straw, a biofuel feedstock, and showed that the range of genes induced was greater than previously seen with simple inducers.
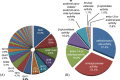
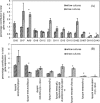
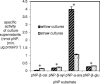
Results: In this work we used RNA-seq to identify the genes induced in A. niger in response to short rotation coppice willow and compared this with the response to wheat straw from our previous study, at the same time-point. The response to willow showed a large increase in expression of genes encoding CAZymes. Genes encoding the major activities required to saccharify lignocellulose were induced on willow such as endoglucanases, cellobiohydrolases and xylanases. The transcriptome response to willow had many similarities with the response to straw with some significant differences in the expression levels of individual genes which are discussed in relation to differences in substrate composition or other factors. Differences in transcript levels include higher levels on wheat straw from genes encoding enzymes classified as members of GH62 (an arabinofuranosidase) and CE1 (a feruloyl esterase) CAZy families whereas two genes encoding endoglucanases classified as members of the GH5 family had higher transcript levels when exposed to willow. There were changes in the cocktail of enzymes secreted by A. niger when cultured with willow or straw. Assays for particular enzymes as well as saccharification assays were used to compare the enzyme activities of the cocktails. Wheat straw induced an enzyme cocktail that saccharified wheat straw to a greater extent than willow. Genes not encoding CAZymes were also induced on willow such as hydrophobins as well as genes of unknown function. Several genes were identified as promising targets for future study.
Conclusions: By comparing this first study of the global transcriptional response of a fungus to willow with the response to straw, we have shown that the inducing lignocellulosic substrate has a marked effect upon the range of transcripts and enzymes expressed by A. niger. The use by industry of complex substrates such as wheat straw or willow could benefit efficient biofuel production.
Keywords: Aspergillus; Biofuels; RNA-seq; Transcriptome; Wheat straw; Willow.
Figures

Similar articles
-
Expression of Aspergillus niger CAZymes is determined by compositional changes in wheat straw generated by hydrothermal or ionic liquid pretreatments.Biotechnol Biofuels. 2017 Feb 7;10:35. doi: 10.1186/s13068-017-0700-9. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 28184248 Free PMC article.
-
RNA-sequencing reveals the complexities of the transcriptional response to lignocellulosic biofuel substrates in Aspergillus niger.Fungal Biol Biotechnol. 2014 Nov 17;1(1):1-14. doi: 10.1186/s40694-014-0003-x. Fungal Biol Biotechnol. 2014. PMID: 26457194 Free PMC article.
-
Genome-wide transcriptional response of Trichoderma reesei to lignocellulose using RNA sequencing and comparison with Aspergillus niger.BMC Genomics. 2013 Aug 9;14:541. doi: 10.1186/1471-2164-14-541. BMC Genomics. 2013. PMID: 24060058 Free PMC article.
-
Modulating Transcriptional Regulation of Plant Biomass Degrading Enzyme Networks for Rational Design of Industrial Fungal Strains.Front Bioeng Biotechnol. 2018 Sep 25;6:133. doi: 10.3389/fbioe.2018.00133. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 30320082 Free PMC article. Review.
-
Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review.Front Bioeng Biotechnol. 2021 Jun 22;9:690773. doi: 10.3389/fbioe.2021.690773. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34239863 Free PMC article. Review.
Cited by
-
Expression of Aspergillus niger CAZymes is determined by compositional changes in wheat straw generated by hydrothermal or ionic liquid pretreatments.Biotechnol Biofuels. 2017 Feb 7;10:35. doi: 10.1186/s13068-017-0700-9. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 28184248 Free PMC article.
-
Aspergillus Hydrophobins: Physicochemical Properties, Biochemical Properties, and Functions in Solid Polymer Degradation.Microorganisms. 2022 Jul 25;10(8):1498. doi: 10.3390/microorganisms10081498. Microorganisms. 2022. PMID: 35893556 Free PMC article. Review.
-
The roles of the zinc finger transcription factors XlnR, ClrA and ClrB in the breakdown of lignocellulose by Aspergillus niger.AMB Express. 2016 Mar;6(1):5. doi: 10.1186/s13568-016-0177-0. Epub 2016 Jan 16. AMB Express. 2016. PMID: 26780227 Free PMC article.
-
Assessing the intracellular primary metabolic profile of Trichoderma reesei and Aspergillus niger grown on different carbon sources.Front Fungal Biol. 2022 Sep 27;3:998361. doi: 10.3389/ffunb.2022.998361. eCollection 2022. Front Fungal Biol. 2022. PMID: 37746225 Free PMC article.
-
Application of carbohydrate arrays coupled with mass spectrometry to detect activity of plant-polysaccharide degradative enzymes from the fungus Aspergillus niger.Sci Rep. 2017 Feb 21;7:43117. doi: 10.1038/srep43117. Sci Rep. 2017. PMID: 28220903 Free PMC article.
References
-
- Delmas S, Pullan ST, Gaddipati S, Kokolski M, Malla S, Blythe MJ, Ibbett R, Campbell M, Liddell S, Aboobaker A, Tucker GA, Archer DB. Uncovering the genome-wide transcriptional responses of the filamentous fungus Aspergillus niger to lignocellulose using RNA sequencing. PLoS Genet. 2012;8:e1002875. doi: 10.1371/journal.pgen.1002875. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

