Effect of celastrol on bone structure and mechanics in arthritic rats
- PMID: 28955491
- PMCID: PMC5604704
- DOI: 10.1136/rmdopen-2017-000438
Effect of celastrol on bone structure and mechanics in arthritic rats
Abstract
Objective: Rheumatoid arthritis (RA) is characterised by chronic inflammation leading to articular bone and cartilage damage. Despite recent progress in RA management, adverse effects, lack of efficacy and economic barriers to treatment access still limit therapeutic success. Therefore, safer and less expensive treatments that control inflammation and bone resorption are needed. We have previously shown that celastrol is a candidate for RA treatment. We have observed that it inhibits both interleukin (IL)-1β and tumor necrosis factor (TNF) in vitro, and that it has anti-inflammatory properties and ability to decrease synovial CD68+ macrophages in vivo. Herein our goal was to evaluate the effect of celastrol in local and systemic bone loss.
Methods: Celastrol was administrated intraperitoneally at a dose of 1 µg/g/day to female Wistar adjuvant-induced arthritic rats. Rats were sacrificed after 22 days of disease progression, and blood, femurs, tibiae and paw samples were collected for bone remodelling markers quantification, 3-point bending test, micro-CT analysis, nanoindentation and Fourier transform infrared spectroscopy measurements, and immunohistochemical evaluation.
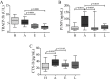
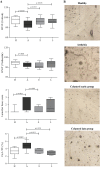
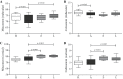
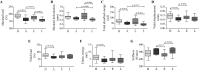
Results: We have observed that celastrol preserved articular structures and decreased the number of osteoclasts and osteoblasts present in arthritic joints. Moreover, celastrol reduced tartrate-resistant acid phosphatase 5b, procollagen type 1 amino-terminal propeptide and C terminal crosslinked telopeptide of type II collagen serum levels. Importantly, celastrol prevented bone loss and bone microarchitecture degradation. Celastrol also preserved bone nanoproperties and mineral content. Additionally, animals treated with celastrol had less fragile bones, as depicted by an increase in maximum load and yield displacement.
Conclusions: These results suggest that celastrol reduces both bone resorption and cartilage degradation, and preserves bone structural properties.
Keywords: Adjuvant-induced arthritis; Bone loss; Celastrol; Inflammation; Rheumatoid arthritis.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
Decrease of CD68 Synovial Macrophages in Celastrol Treated Arthritic Rats.PLoS One. 2015 Dec 11;10(12):e0142448. doi: 10.1371/journal.pone.0142448. eCollection 2015. PLoS One. 2015. PMID: 26658436 Free PMC article.
-
Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis.Arthritis Res. 1999;1(1):81-91. doi: 10.1186/ar14. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11056663 Free PMC article.
-
Celastrol Efficacy by Oral Administration in the Adjuvant-Induced Arthritis Model.Front Med (Lausanne). 2020 Sep 8;7:455. doi: 10.3389/fmed.2020.00455. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33015082 Free PMC article.
-
Effective treatment of rat adjuvant-induced arthritis by celastrol.Autoimmun Rev. 2012 Oct;11(12):856-62. doi: 10.1016/j.autrev.2012.02.022. Epub 2012 Mar 3. Autoimmun Rev. 2012. PMID: 22415021 Free PMC article.
-
Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases.Front Med (Lausanne). 2017 Jun 15;4:69. doi: 10.3389/fmed.2017.00069. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28664158 Free PMC article. Review.
Cited by
-
A microfluidic chip-based co-culture of fibroblast-like synoviocytes with osteoblasts and osteoclasts to test bone erosion and drug evaluation.R Soc Open Sci. 2018 Sep 12;5(9):180528. doi: 10.1098/rsos.180528. eCollection 2018 Sep. R Soc Open Sci. 2018. PMID: 30839692 Free PMC article.
-
Chondro-protective effects of celastrol on osteoarthritis through autophagy activation and NF-κB signaling pathway inhibition.Inflamm Res. 2020 Apr;69(4):385-400. doi: 10.1007/s00011-020-01327-z. Epub 2020 Feb 28. Inflamm Res. 2020. PMID: 32112120
-
Celastrol Pyrazine Derivative Alleviates Silicosis Progression via Inducing ROS-Mediated Apoptosis in Activated Fibroblasts.Molecules. 2024 Jan 22;29(2):538. doi: 10.3390/molecules29020538. Molecules. 2024. PMID: 38276616 Free PMC article.
-
Rheumatoid arthritis-recent advances in pathogenesis and the anti-inflammatory effect of plant-derived COX inhibitors.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):5363-5385. doi: 10.1007/s00210-024-02982-3. Epub 2024 Feb 15. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38358467 Review.
-
Celastrol protects against cisplatin-induced ovarian toxicity via modulation of PPAR-γ and AMPK.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun 7. doi: 10.1007/s00210-025-04322-5. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40481255
References
-
- Haugeberg G, Uhlig T, Falch JA, et al. . Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum 2000;43:522–30. 10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
