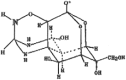
Xeronine structure and function: computational comparative mastery of its mystery
- PMID: 28955650
- PMCID: PMC5581797
- DOI: 10.1007/s40203-017-0028-y
Xeronine structure and function: computational comparative mastery of its mystery
Abstract
Morinda citrifolia (Noni) fruit has a long history of dietary use in tropical regions of the world. Pharmacological properties that have been attributed to the fruit include anti-inflammatory, anti-cancer, and antioxidant properties. Xeronine, a small alkaloid which has been patented (US4543212) is one of the bioactive compounds of Noni fruit, which is believed to be capable of modifying the molecular structure of specific inactive proteins thereby regulating proper folding to active enzymes. Despite reports of the potential of Xeronine as therapeutic agent, its presence is controversial and its structure has not been explored. In this study, standard chemoinformatics tools and servers such as ChemSketch, ChemMine, Swisstargetprediction, SwissADME and Swisssimilarity have been employed to predict its possible structure. In addition, synthetic xeronine structures based on the known bioactive components of Noni fruit were designed. Results showed that the hypothetical structure of xeronine provided by the patent inventor is a mystery based on its <5% probable protein targets and no similarity match to the US Food and Drug Administration (FDA) approved drugs and experimental compounds by in silico evaluation. By constrast, final designed xeronine structure possess all the features that were described in the patent document, and has >40% probable protein targets related to neurodegenerative diseases such as Alzheimer's disease (AD), which possibly justifies the key function stated in the patent.
Keywords: Cheminformatics; De novo drug design; Morinda citrifolia; Noni fruit; Xeronine.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Morinda citrifolia (Noni) fruit--phytochemistry, pharmacology, safety.Planta Med. 2007 Mar;73(3):191-9. doi: 10.1055/s-2007-967115. Epub 2007 Feb 7. Planta Med. 2007. PMID: 17286240 Review.
-
New constituents from noni (Morinda citrifolia) fruit juice.J Agric Food Chem. 2006 Aug 23;54(17):6398-402. doi: 10.1021/jf060672u. J Agric Food Chem. 2006. PMID: 16910736
-
Anticancer activity of Morinda citrifolia (Noni) fruit: a review.Phytother Res. 2012 Oct;26(10):1427-40. doi: 10.1002/ptr.4595. Epub 2012 Feb 17. Phytother Res. 2012. PMID: 22344842 Review.
-
[Literature research and discussion of Chinese medicinal properties of Morinda citrifolia].Zhongguo Zhong Yao Za Zhi. 2020 Mar;45(5):984-990. doi: 10.19540/j.cnki.cjcmm.20191230.403. Zhongguo Zhong Yao Za Zhi. 2020. PMID: 32237436 Chinese.
-
Antitumour potential of a polysaccharide-rich substance from the fruit juice of Morinda citrifolia (Noni) on sarcoma 180 ascites tumour in mice.Phytother Res. 2003 Dec;17(10):1158-64. doi: 10.1002/ptr.1307. Phytother Res. 2003. PMID: 14669249
Cited by
-
Anti-Alopecia Activity of Alkaloids Group from Noni Fruit against Dihydrotestosterone-Induced Male Rabbits and Its Molecular Mechanism: In Vivo and In Silico Studies.Pharmaceuticals (Basel). 2022 Dec 14;15(12):1557. doi: 10.3390/ph15121557. Pharmaceuticals (Basel). 2022. PMID: 36559008 Free PMC article.
-
Reverse Screening Methods to Search for the Protein Targets of Chemopreventive Compounds.Front Chem. 2018 May 9;6:138. doi: 10.3389/fchem.2018.00138. eCollection 2018. Front Chem. 2018. PMID: 29868550 Free PMC article. Review.
-
Secondary Metabolites of Plants as Modulators of Endothelium Functions.Int J Mol Sci. 2021 Mar 3;22(5):2533. doi: 10.3390/ijms22052533. Int J Mol Sci. 2021. PMID: 33802468 Free PMC article. Review.
-
Computational Approaches in Preclinical Studies on Drug Discovery and Development.Front Chem. 2020 Sep 11;8:726. doi: 10.3389/fchem.2020.00726. eCollection 2020. Front Chem. 2020. PMID: 33062633 Free PMC article. Review.
-
Investigation of Morinda citrifolia Activities through Pinoresinol and α-EG Related Gene Expression.Plants (Basel). 2022 Jul 30;11(15):1985. doi: 10.3390/plants11151985. Plants (Basel). 2022. PMID: 35956463 Free PMC article.
References
-
- Agudo A, Ribeiro JM, Canales J, Cameselle JC. Use of potato tuber nucleotide pyrophosphatase to synthesize adenosine 5′-monophosphate methyl ester: evidence that the solvolytic preferences of the enzyme are regulated by pH and temperature. Biotechnol Bioeng. 1998;59(1):62–67. doi: 10.1002/(SICI)1097-0290(19980705)59:1<62::AID-BIT8>3.0.CO;2-R. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources