Oncolytic Viruses-Natural and Genetically Engineered Cancer Immunotherapies
- PMID: 28955655
- PMCID: PMC5600978
- DOI: 10.3389/fonc.2017.00202
Oncolytic Viruses-Natural and Genetically Engineered Cancer Immunotherapies
Abstract
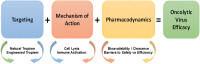
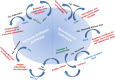
There has long been interest in innovating an approach by which tumor cells can be selectively and specifically targeted and destroyed. The discovery of viruses that lyse tumor cells, termed oncolytic viruses (OVs), has led to a revolution in the treatment of cancer. The potential of OVs to improve the therapeutic ratio is derived from their ability to preferentially infect and replicate in cancer cells while avoiding destruction of normal cells surrounding the tumor. Two main mechanisms exist through which these viruses are reported to improve outcomes: direct lysis of tumor cells and indirect augmentation of host anti-tumor immunity. With these factors in mind, viruses are chosen or modified to selectively target tumor cells, decrease pathogenicity to normal cells, decrease the antiviral immune response (to prevent viral clearance), and increase the antitumor immune response. While only one OV has been approved for the treatment of cancer in the United States, and only two other OVs have been approved worldwide, a wide spectrum of OVs are in various stages of preclinical development and in clinical trials. These viruses are being studied as alternatives and adjuncts to more traditional cancer therapies including surgical resection, chemotherapy, radiation, hormonal therapies, targeted therapies, and other immunotherapies. Here, we review the natural characteristics and genetically engineered modifications that enhance the effectiveness of OVs for the treatment of cancer.
Keywords: cancer immunotherapy; oncoimmunology; oncolytic viruses; pathogens; viruses.
Figures
References
-
- Smith AJ, Oertle J, Prato D. Immunotherapy in cancer treatment. Open J Med Microbiol (2014) 4(03):178.10.4236/ojmm.2014.43020 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

