Nitric oxide inhibits ATPase activity and induces resistance to topoisomerase II-poisons in human MCF-7 breast tumor cells
- PMID: 28955753
- PMCID: PMC5614683
- DOI: 10.1016/j.bbrep.2017.04.011
Nitric oxide inhibits ATPase activity and induces resistance to topoisomerase II-poisons in human MCF-7 breast tumor cells
Abstract
Background: Topoisomerase poisons are important drugs for the management of human malignancies. Nitric oxide (•NO), a physiological signaling molecule, induces nitrosylation (or nitrosation) of many cellular proteins containing cysteine thiol groups, altering their cellular functions. Topoisomerases contain several thiol groups which are important for their activity and are also targets for nitrosation by nitric oxide.
Methods: Here, we have evaluated the roles of • NO/ • NO-derived species in the stability and activity of topo II (α and β) both in vitro and in human MCF-7 breast tumor cells. Furthermore, we have examined the effects of • NO on the ATPase activity of topo II.
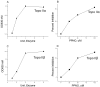
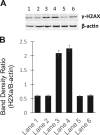
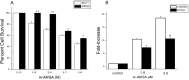
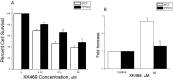
Results: Treatment of purified topo IIα and β with propylamine propylamine nonoate (PPNO), an NO donor, resulted in inhibition of the catalytic activity of topo II. Furthermore, PPNO significantly inhibited topo II-dependent ATP hydrolysis. • NO-induced inhibition of these topo II (α and β) functions resulted in a decrease in cleavable complex formation in MCF-7 cells in the presence of m-AMSA and XK469 and induced significant resistance to both drugs in MCF-7 cells.
Conclusion: PPNO treatment resulted in the nitrosation of the topo II protein in MCF-7 cancer cells and inhibited both catalytic-, and ATPase activities of topo II. Furthermore, PPNO significantly affected the DNA damage and cytotoxicity of m-AMSA and XK469 in MCF-7 tumor cells.
General significance: As tumors express nitric oxide synthase and generate • NO, inhibition of topo II functions by • NO/ • NO-derived species could render tumors resistant to certain topo II-poisons in the clinic.
Keywords: ATPase inhibition; Nitric oxide; Resistance; Topoisomerase; XK469; m-AMSA.
Figures


Similar articles
-
Nitric oxide inhibits topoisomerase II activity and induces resistance to topoisomerase II-poisons in human tumor cells.Biochim Biophys Acta. 2016 Jul;1860(7):1519-27. doi: 10.1016/j.bbagen.2016.04.009. Epub 2016 Apr 17. Biochim Biophys Acta. 2016. PMID: 27095671 Free PMC article.
-
Nitric Oxide Down-Regulates Topoisomerase I and Induces Camptothecin Resistance in Human Breast MCF-7 Tumor Cells.PLoS One. 2015 Nov 5;10(11):e0141897. doi: 10.1371/journal.pone.0141897. eCollection 2015. PLoS One. 2015. PMID: 26540186 Free PMC article.
-
2-[4-(7-chloro-2-quinoxalinyloxyphenoxy]-propionic acid (XK469), an inhibitor of topoisomerase (Topo) IIbeta, up-regulates Topo IIalpha and enhances Topo IIalpha-mediated cytotoxicity.Mol Cancer Ther. 2002 Dec;1(14):1321-6. Mol Cancer Ther. 2002. PMID: 12516965
-
Recent advances in the development of dual topoisomerase I and II inhibitors as anticancer drugs.Curr Med Chem. 2010;17(35):4270-90. doi: 10.2174/092986710793361252. Curr Med Chem. 2010. PMID: 20939813 Review.
-
DNA topoisomerase I and II as targets for rational design of new anticancer drugs.Ann Oncol. 1993 Aug;4(7):533-43. doi: 10.1093/oxfordjournals.annonc.a058584. Ann Oncol. 1993. PMID: 8395870 Review.
Cited by
-
Can Nitric Oxide-Based Therapy Be Improved for the Treatment of Cancers? A Perspective.Int J Mol Sci. 2023 Sep 2;24(17):13611. doi: 10.3390/ijms241713611. Int J Mol Sci. 2023. PMID: 37686417 Free PMC article.
-
NCX-4040, a Unique Nitric Oxide Donor, Induces Reversal of Drug-Resistance in Both ABCB1- and ABCG2-Expressing Multidrug Human Cancer Cells.Cancers (Basel). 2021 Apr 2;13(7):1680. doi: 10.3390/cancers13071680. Cancers (Basel). 2021. PMID: 33918289 Free PMC article.
-
Role of Oxygen and Nitrogen Radicals in the Mechanism of Anticancer Drug Cytotoxicity.J Cancer Sci Ther. 2020;12(1):10-18. Epub 2020 Jan 24. J Cancer Sci Ther. 2020. PMID: 32494339 Free PMC article.
-
Glycyrrhizin as a Nitric Oxide Regulator in Cancer Chemotherapy.Cancers (Basel). 2021 Nov 17;13(22):5762. doi: 10.3390/cancers13225762. Cancers (Basel). 2021. PMID: 34830916 Free PMC article. Review.
-
Nitric oxide reverses drug resistance by inhibiting ATPase activity of p-glycoprotein in human multi-drug resistant cancer cells.Biochim Biophys Acta Gen Subj. 2018 Dec;1862(12):2806-2814. doi: 10.1016/j.bbagen.2018.08.021. Epub 2018 Sep 1. Biochim Biophys Acta Gen Subj. 2018. PMID: 30251669 Free PMC article.
References
-
- Murad F. Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone? Recent Prog. Horm. Res. 1998;53:43–59. (discussion 59–60) - PubMed
-
- Murad F. Shattuck lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006;355:2003–2011. - PubMed
-
- Hirst D., Robson T. Nitric oxide in cancer therapeutics: interaction with cytotoxic chemotherapy. Curr. Pharm. Des. 2010;16:411–420. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

