Isothermal titration calorimetry uncovers substrate promiscuity of bicupin oxalate oxidase from Ceriporiopsis subvermispora
- PMID: 28955847
- PMCID: PMC5600335
- DOI: 10.1016/j.bbrep.2016.01.016
Isothermal titration calorimetry uncovers substrate promiscuity of bicupin oxalate oxidase from Ceriporiopsis subvermispora
Abstract
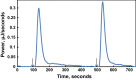
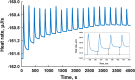
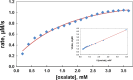
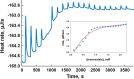
Isothermal titration calorimetry (ITC) may be used to determine the kinetic parameters of enzyme-catalyzed reactions when neither products nor reactants are spectrophotometrically visible and when the reaction products are unknown. We report here the use of the multiple injection method of ITC to characterize the catalytic properties of oxalate oxidase (OxOx) from Ceriporiopsis subvermispora (CsOxOx), a manganese dependent enzyme that catalyzes the oxygen-dependent oxidation of oxalate to carbon dioxide in a reaction coupled with the formation of hydrogen peroxide. CsOxOx is the first bicupin enzyme identified that catalyzes this reaction. The multiple injection ITC method of measuring OxOx activity involves continuous, real-time detection of the amount of heat generated (dQ) during catalysis, which is equal to the number of moles of product produced times the enthalpy of the reaction (ΔHapp). Steady-state kinetic constants using oxalate as the substrate determined by multiple injection ITC are comparable to those obtained by a continuous spectrophotometric assay in which H2O2 production is coupled to the horseradish peroxidase-catalyzed oxidation of 2,2'-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) and by membrane inlet mass spectrometry. Additionally, we used multiple injection ITC to identify mesoxalate as a substrate for the CsOxOx-catalyzed reaction, with a kinetic parameters comparable to that of oxalate, and to identify a number of small molecule carboxylic acid compounds that also serve as substrates for the enzyme.
Keywords: ABTS, 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid).; CsOxOx, OxOx from Ceriporiopsis subvermispora; Cupin; Enzyme kinetics; HRP, horseradish peroxidase; Isothermal titration calorimetry; OxOx, oxalate oxidase; Oxalate oxidase.
Figures
Similar articles
-
Hydrogen peroxide inhibition of bicupin oxalate oxidase.PLoS One. 2017 May 9;12(5):e0177164. doi: 10.1371/journal.pone.0177164. eCollection 2017. PLoS One. 2017. PMID: 28486485 Free PMC article.
-
Membrane inlet mass spectrometry reveals that Ceriporiopsis subvermispora bicupin oxalate oxidase is inhibited by nitric oxide.Biochem Biophys Res Commun. 2014 Jul 18;450(1):750-4. doi: 10.1016/j.bbrc.2014.06.040. Epub 2014 Jun 19. Biochem Biophys Res Commun. 2014. PMID: 24953692
-
Characterization of Ceriporiopsis subvermispora bicupin oxalate oxidase expressed in Pichia pastoris.Arch Biochem Biophys. 2011 May 1;509(1):100-7. doi: 10.1016/j.abb.2011.02.022. Epub 2011 Mar 2. Arch Biochem Biophys. 2011. PMID: 21376010 Free PMC article.
-
Isothermal titration calorimetry: experimental design, data analysis, and probing macromolecule/ligand binding and kinetic interactions.Methods Cell Biol. 2008;84:79-113. doi: 10.1016/S0091-679X(07)84004-0. Methods Cell Biol. 2008. PMID: 17964929 Review.
-
Enzyme Kinetics by Isothermal Titration Calorimetry: Allostery, Inhibition, and Dynamics.Front Mol Biosci. 2020 Oct 19;7:583826. doi: 10.3389/fmolb.2020.583826. eCollection 2020. Front Mol Biosci. 2020. PMID: 33195429 Free PMC article. Review.
Cited by
-
Quantifying the flux as the driving force for nonequilibrium dynamics and thermodynamics in non-Michaelis-Menten enzyme kinetics.Proc Natl Acad Sci U S A. 2020 Jan 14;117(2):923-930. doi: 10.1073/pnas.1819572117. Epub 2019 Dec 26. Proc Natl Acad Sci U S A. 2020. PMID: 31879351 Free PMC article.
-
Carbon Nanotube PtSn Nanoparticles for Enhanced Complete Biocatalytic Oxidation of Ethylene Glycol in Biofuel Cells.ACS Mater Au. 2021 Oct 18;2(2):94-102. doi: 10.1021/acsmaterialsau.1c00029. eCollection 2022 Mar 9. ACS Mater Au. 2021. PMID: 36855769 Free PMC article.
-
Hydrogen peroxide inhibition of bicupin oxalate oxidase.PLoS One. 2017 May 9;12(5):e0177164. doi: 10.1371/journal.pone.0177164. eCollection 2017. PLoS One. 2017. PMID: 28486485 Free PMC article.
References
-
- Makela M.R., Donofrio N., de Vries R.P. Plant biomass degradation by fungi. Fungal Genet. Biol. 2014;72:2–9. - PubMed
-
- Giles R.L., Zackeru J.C., Galloway E.R., Elliott G.D., Parrow M.W. Single versus simultaneous species treatment of wood with Ceriporiopsis subvermispora and Postia placenta for ethanol applications, with observations on interspecific growth inhibition. Int. Biodeterior. Biodegrad. 2015;99:66–72.
-
- Cianchetta S., Di Maggio B., Burzi P.L., Galletti S. Evaluation of selected white-rot fungal isolates for improving the sugar yield from wheat straw. Appl. Biochem. Biotechnol. 2014;173:609–623. - PubMed
-
- Zhao J., Ge X.M., Vasco-Correa J., Li Y.B. Fungal pretreatment of unsterilized yard trimmings for enhanced methane production by solid-state anaerobic digestion. Bioresour. Technol. 2014;158:248–252. - PubMed
-
- Shimazono H. Oxalic acid decarboxylase, a new enzyme from the mycelium of wood destroying fungi. J. Biochem. 1955;42:321–340.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

