Enhancement of antitumor effect by peptide vaccine therapy in combination with anti-CD4 antibody: Study in a murine model
- PMID: 28955856
- PMCID: PMC5600353
- DOI: 10.1016/j.bbrep.2016.02.010
Enhancement of antitumor effect by peptide vaccine therapy in combination with anti-CD4 antibody: Study in a murine model
Abstract
Purpose: The clinical efficacy of cancer peptide vaccine therapy is insufficient. To enhance the anti-tumor effect of peptide vaccine therapy, we combined this therapy with an anti-CD4 mAb (GK1.5), which is known to deplete CD4+ cells, including regulatory T cells (Tregs).
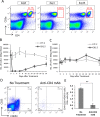
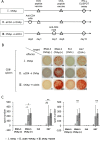
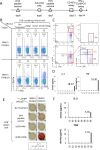
Methods: To determine the treatment schedule, the number of lymphocyte subsets in the peripheral blood of mice was traced by flow cytometry after administration of anti-CD4 mAb. The ovalbumin (OVA)257-264 peptide vaccine was injected intradermally and anti-CD4 mAb was administered intraperitoneally into C57BL/6 mice at different schedules. We evaluated the enhancement of OVA peptide-specific cytotoxic T lymphocyte (CTL) induction in the combination therapy using the ELISPOT assay, CD107a assay, and cytokine assay. We then examined the in vivo metastasis inhibitory effect by OVA peptide vaccine therapy in combination with anti-CD4 mAb against OVA-expressing thymoma (EG7) in a murine liver metastatic model.
Results: We showed that peptide-specific CTL induction was enhanced by the peptide vaccine in combination with anti-CD4 mAb and that the optimized treatment schedule had the strongest induction effect of peptide-specific CTLs using an IFN-γ ELISPOT assay. We also confirmed that the CD107a+ cells secreted perforin and granzyme B and the amount of IL-2 and TNF produced by these CTLs increased when the peptide vaccine was combined with anti-CD4 mAb. Furthermore, metastasis was inhibited by peptide vaccines in combination with anti-CD4 mAb compared to peptide vaccine alone in a murine liver metastatic model.
Conclusion: The use of anti-CD4 mAb in combination with the OVA peptide vaccine therapy increased the number of peptide-specific CTLs and showed a higher therapeutic effect against OVA-expressing tumors. The combination with anti-CD4 mAb may provide a new cancer vaccine strategy.
Keywords: 7-AAD, 7-amino-actinomycin D; Anti-CD4 antibody; CTL, cytotoxic T lymphocyte; Cancer; DC, dendritic cell; ELISPOT assay, enzyme-linked immunospot assay; FITC, fluorescein isothiocyanate; FOXP3, forkhead box P3; GPC3, glypican-3; HCC, hepatocellular carcinoma; IFN-γ, interferon-γ; IL-2, interleukine-2; Immunotherapy; MHC, major histocompatibility complex; Murine liver metastatic model; OVA, ovalbumin; PD-1, programmed death-1; PE, phycoerythrin; Peptide vaccine; QOL, quality of life; TGF-β, transforming growth factor-βl; TNF, tumor necrosis factor; Treg, regulatory T cell; mAb, monoclonal antibody.
Figures


Similar articles
-
Combination therapy with dendritic cell vaccine and IL-2 encapsulating polymeric micelles enhances intra-tumoral accumulation of antigen-specific CTLs.Int Immunopharmacol. 2014 Dec;23(2):499-504. doi: 10.1016/j.intimp.2014.09.025. Epub 2014 Oct 3. Int Immunopharmacol. 2014. PMID: 25284343
-
Impact of anti-CD25 monoclonal antibody on dendritic cell-tumor fusion vaccine efficacy in a murine melanoma model.J Transl Med. 2013 Jun 17;11:148. doi: 10.1186/1479-5876-11-148. J Transl Med. 2013. PMID: 23768240 Free PMC article.
-
Dendritic cells engineered to secrete anti-DcR3 antibody augment cytotoxic T lymphocyte response against pancreatic cancer in vitro.World J Gastroenterol. 2017 Feb 7;23(5):817-829. doi: 10.3748/wjg.v23.i5.817. World J Gastroenterol. 2017. PMID: 28223726 Free PMC article.
-
Transient depletion of CD4(+) T cells augments IL-21-based immunotherapy of disseminated neuroblastoma in syngeneic mice.Int J Cancer. 2010 Sep 1;127(5):1141-50. doi: 10.1002/ijc.25140. Int J Cancer. 2010. PMID: 20039320
-
Pro-inflammatory responses after peptide-based cancer immunotherapy.Heliyon. 2024 May 31;10(11):e32249. doi: 10.1016/j.heliyon.2024.e32249. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38912474 Free PMC article. Review.
Cited by
-
IL-6, IL-17 and Stat3 are required for auto-inflammatory syndrome development in mouse.Sci Rep. 2018 Oct 25;8(1):15783. doi: 10.1038/s41598-018-34173-5. Sci Rep. 2018. PMID: 30361689 Free PMC article.
-
Cancer immunotherapy-targeted glypican-3 or neoantigens.Cancer Sci. 2018 Mar;109(3):531-541. doi: 10.1111/cas.13485. Epub 2018 Feb 14. Cancer Sci. 2018. PMID: 29285841 Free PMC article. Review.
-
Screening of novel tumor-associated antigens for lung adenocarcinoma mRNA vaccine development based on pyroptosis phenotype genes.BMC Cancer. 2024 Jan 2;24(1):28. doi: 10.1186/s12885-023-11757-7. BMC Cancer. 2024. PMID: 38166691 Free PMC article.
-
Bidirectional crosstalk between therapeutic cancer vaccines and the tumor microenvironment: Beyond tumor antigens.Fundam Res. 2022 Mar 26;3(6):1005-1024. doi: 10.1016/j.fmre.2022.03.009. eCollection 2023 Nov. Fundam Res. 2022. PMID: 38933006 Free PMC article. Review.
-
Identification of tumor antigens and immune subtypes in lower grade gliomas for mRNA vaccine development.J Transl Med. 2021 Aug 17;19(1):352. doi: 10.1186/s12967-021-03014-x. J Transl Med. 2021. PMID: 34404444 Free PMC article.
References
-
- Nakatsura T., Yoshitake Y., Senju S. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem. Biophys. Res. Commun. 2003;306:16–25. - PubMed
-
- Shirakawa H., Kuronuma T., Nishimura Y. Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver Cancer. Int. J. Oncol. 2009;34:649–656. - PubMed
-
- Komori H., Nakatsura T., Senju S. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin. Cancer Res. 2006;12:2689–2697. - PubMed
-
- Nakatsura T., Komori H., Kubo T. Mouse homologue of a novel human oncofetal antigen, glypican-3, evokes T-cell-mediated tumor rejection without autoimmune reactions in mice. Clin. Cancer Res. 2004;10:8630–8640. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

