An efficient method for the preparation of preferentially heterodimerized recombinant S100A8/A9 coexpressed in Escherichia coli
- PMID: 28955868
- PMCID: PMC5600424
- DOI: 10.1016/j.bbrep.2016.03.009
An efficient method for the preparation of preferentially heterodimerized recombinant S100A8/A9 coexpressed in Escherichia coli
Abstract
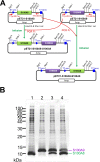
It is now known that multicomponent protein assemblies strictly regulate many protein functions. The S100 protein family is known to play various physiological roles, which are associated with alternative complex formations. To prepare sufficient amounts of heterodimeric S100A8 and S100A9 proteins, we developed a method for bicistronic coexpression from a single-vector system using Escherichia coli cells as a host. The complex formation between S100A8 and S100A9 appears to be dependent on the thermodynamic stability of the protein during expression. The stable S100A8/A9 heterodimer complex spontaneously formed during coexpression, and biologically active samples were purified by cation-exchange chromatography. Semi-stable homodimers of S100A8 and S100A9 were also formed when expressed individually. These results suggest that the assembly of S100 protein complexes might be regulated by expression levels of partner proteins in vivo. Because protein assembly occurs rapidly after protein synthesis, coexpression of relevant proteins is crucial for the design of multicomponent recombinant protein expression systems.
Keywords: Calprotectin; Coexpression; Heterodimerization; S100 protein.
Figures



Similar articles
-
S100A8, S100A9 and the S100A8/A9 heterodimer complex specifically bind to human endothelial cells: identification and characterization of ligands for the myeloid-related proteins S100A9 and S100A8/A9 on human dermal microvascular endothelial cell line-1 cells.Int Immunol. 2002 Mar;14(3):287-97. doi: 10.1093/intimm/14.3.287. Int Immunol. 2002. PMID: 11867565
-
[Possibility of formation of the S100A8/A9-proinflammatory cytokine complexes in vivo in acute inflammation and their functional roles].Rinsho Byori. 2009 Apr;57(4):324-31. Rinsho Byori. 2009. PMID: 19489433 Japanese.
-
High Amounts of S100-Alarmins Confer Antimicrobial Activity on Human Breast Milk Targeting Pathogens Relevant in Neonatal Sepsis.Front Immunol. 2017 Dec 13;8:1822. doi: 10.3389/fimmu.2017.01822. eCollection 2017. Front Immunol. 2017. PMID: 29326708 Free PMC article.
-
Calprotectin and the Initiation and Progression of Head and Neck Cancer.J Dent Res. 2018 Jun;97(6):674-682. doi: 10.1177/0022034518756330. Epub 2018 Feb 14. J Dent Res. 2018. PMID: 29443623 Free PMC article. Review.
-
S100A8/A9 in Inflammation.Front Immunol. 2018 Jun 11;9:1298. doi: 10.3389/fimmu.2018.01298. eCollection 2018. Front Immunol. 2018. PMID: 29942307 Free PMC article. Review.
Cited by
-
Calprotectin-Mediated Zinc Chelation Inhibits Pseudomonas aeruginosa Protease Activity in Cystic Fibrosis Sputum.J Bacteriol. 2021 Jun 8;203(13):e0010021. doi: 10.1128/JB.00100-21. Epub 2021 Jun 8. J Bacteriol. 2021. PMID: 33927050 Free PMC article.
-
Extracellular S100A11 Plays a Critical Role in Spread of the Fibroblast Population in Pancreatic Cancers.Oncol Res. 2019 Jun 21;27(6):713-727. doi: 10.3727/096504018X15433161908259. Epub 2019 Mar 8. Oncol Res. 2019. PMID: 30850029 Free PMC article.
-
Evolution of multifunctionality through a pleiotropic substitution in the innate immune protein S100A9.Elife. 2020 Apr 7;9:e54100. doi: 10.7554/eLife.54100. Elife. 2020. PMID: 32255429 Free PMC article.
-
Generation of a novel monoclonal antibody against inflammatory biomarker S100A8 using hybridoma technology.Biotechnol Lett. 2023 Jun;45(5-6):589-600. doi: 10.1007/s10529-023-03364-0. Epub 2023 Mar 27. Biotechnol Lett. 2023. PMID: 36971774
-
The heterodimer S100A8/A9 is a potent therapeutic target for idiopathic pulmonary fibrosis.J Mol Med (Berl). 2021 Jan;99(1):131-145. doi: 10.1007/s00109-020-02001-x. Epub 2020 Nov 9. J Mol Med (Berl). 2021. PMID: 33169236
References
-
- Hessian P.A., Fisher L. The heterodimeric complex of MRP-8 (S100A8) and MRP-14 (S100A9). Antibody recognition, epitope definition and the implications for structure. Eur. J. Biochem. 2001;268:353–363. - PubMed
-
- Gebhardt C., Breitenbach U., Tuckermann J.P., Dittrich B.T., Richter K.H., Angel P. Calgranulins S100A8 and S100A9 are negatively regulated by glucocorticoids in a c-Fos-dependent manner and overexpressed throughout skin carcinogenesis. Oncogene. 2002;21:4266–4276. - PubMed
-
- Itou H., Yao M., Fujita I., Watanabe N., Suzuki M., Nishihira J., Tanaka I. The crystal structure of human MRP14 (S100A9), a Ca(2+)-dependent regulator protein in inflammatory process. J. Mol. Biol. 2002;316:265–276. - PubMed
-
- Gebhardt C., Németh J., Angel P., Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem. Pharm. 2006;72:1622–1631. - PubMed
-
- Ehrchen J.M., Sunderkötter C., Foell D., Vogl T., Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009;86:557–566. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

