N-acetyl-L-methionine is a superior protectant of human serum albumin against post-translational oxidation as compared to N-acetyl-L-tryptophan
- PMID: 28955884
- PMCID: PMC5600351
- DOI: 10.1016/j.bbrep.2016.04.011
N-acetyl-L-methionine is a superior protectant of human serum albumin against post-translational oxidation as compared to N-acetyl-L-tryptophan
Abstract
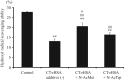
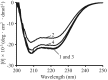
Sodium octanoate and N-acetyl-L-tryptophan (N-AcTrp) are widely used as stabilizers during pasteurization and storage of albumin products. However, as compared with N-AcTrp, N-acetyl-L-methionine (N-AcMet) is superior in protecting albumin exposed to light during storage. Here, we examine, whether N-AcMet also is better than N-AcTrp to protect albumin against oxidation. Recombinant human serum albumin (rHSA) without and with N-AcMet or N-AcTrp was oxidized by using chloramine-T (CT) as a model compound for mimicking oxidative stress. Oxidation of rHSA was examined by determining carbonyl groups and advanced oxidation protein products. Structural changes were studied by native-PAGE, circular dichroism, intrinsic fluorescence and differential scanning calorimetry. The anti-oxidant capacity of CT-treated rHSA was quantified by its ability to scavenge peroxynitrite and the hydroxyl radical. The pharmacokinetics of indocyanine green-labeled albumin preparations was studied in male mice. We found that the number of chemical modifications and the structural changes of rHSA were significantly smaller in the presence of N-AcMet than in the presence of N-AcTrp. The anti-oxidant properties of CT-exposed rHSA were best protected by adding N-AcMet. Finally, N-AcMet is superior in preserving the normal pharmacokinetics of rHSA. Thus, N-AcMet is superior to N-AcTrp in protecting albumin preparations against oxidation. In addition, N-AcMet is probable also useful for protecting other proteins. Therefore, N-AcMet should be useful as a new and effective stabilizer and antioxidant for albumin isolated from blood, rHSA, albumin-fusion proteins and for preparations of rHSA-therapeutic complexes.
Keywords: CD, circular dichroism; CT, chloramine-T; DSC, differential scanning calorimetry; HSA, human serum albumin; Human serum albumin; N-AcMet, N-acetyl-L-methionine; N-AcTrp, N-acetyl-L-tryptophan; N-Acetyl-L-Methionine; N-Acetyl-L-Tryptophan; Oct, octanoate; Oxidation; Pharmacokinetics; ROS, reactive oxygen species; Structural Changes; rHSA, recombinant HSA.
Figures







References
-
- Peters T., Jr. Academic Press,; San Diego, California: 1996. All about Albumin: Biochemistry Genetics, and Medical Applications.
-
- Garcia-Martinez R., Noiret L., Sen S., Mookerjee R., Jalan R. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Liver Int. 2015;35:335–343. - PubMed
-
- Yamasaki K., Chuang V.T., Maruyama T., Otagiri M. Albumin-drug interaction and its clinical implication. Biochim. Biophys. Acta. 1830;2013:5435–5443. - PubMed
-
- Sato H., Chuang V.T., Yamasaki K., Yamaotsu N., Watanabe H., Nagumo K., Anraku M., Kadowaki D., Ishima Y., Hirono S., Otagiri M., Maruyama T. Differential effects of methoxy group on the interaction of curcuminoids with two major ligand binding sites of human serum albumin. PLoS. One. 2014;9:e87919. - PMC - PubMed
-
- Anraku M., Chuang V.T., Maruyama T., Otagiri M. Redox properties of serum albumin. Biochim. Biophys. Acta. 1830;2013:5465–5472. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

