Actin exposure upon tissue injury is a targetable wound site-specific protein marker
- PMID: 28955889
- PMCID: PMC5613279
- DOI: 10.1016/j.bbrep.2016.05.013
Actin exposure upon tissue injury is a targetable wound site-specific protein marker
Abstract
Background: Identification of wound-specific markers would represent an important step toward damaged tissue detection and targeted delivery of biologically important materials to injured sites. Such delivery could minimize the amount of therapeutic materials that must be administered and limit potential collateral damage on nearby normal tissues. Yet, biological markers that are specific for injured tissue sites remain elusive.
Methods: In this study, we have developed an immunohistological approach for identification of protein epitopes specifically exposed in wounded tissue sites.
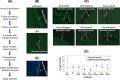
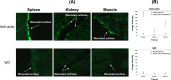
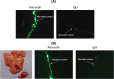
Results: Using ex-vivo tissue samples in combination with fluorescently-labeled antibodies we show that actin, an intracellular cytoskeletal protein, is specifically exposed upon injury. The targetability of actin in injured sites has been demonstrated in vivo through the specific delivery of anti-actin conjugated particles to the wounded tissue in a lethal rat model of grade IV liver injury.
Conclusions: These results illustrate that identification of injury-specific protein markers and their targetability for specific delivery is feasible.
General significance: Identification of wound-specific targets has important medical applications as it could enable specific delivery of various products, such as expression vectors, therapeutic drugs, hemostatic materials, tissue healing, or scar prevention agents, to internal sites of penetrating or surgical wounds regardless of origin, geometry or location.
Keywords: Actin; Bleeding; Hemorrhage; Injury; Protein marker; Wound.
Figures

References
-
- Agnihotri S.A., Mallikarjuna N.N., Aminabhavi T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release. 2004;100:5–28. - PubMed
-
- Ahrens S., Zelenay S., Sancho D., Hanč P., Kjær S. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity. 2012;36:635–645. - PubMed
-
- Emoto K., Nagasaki Y., Iijima M., Kato M., Kataoka K. Preparation of non-fouling surface through the coating with core-polymerized block copolymer micelles having aldehyde-ended PEG shell. Colloids Surf. B: Biointerfaces. 2000;18:337–346. - PubMed
-
- Gasper M.M., Blanco D., Cruz M.E., Alonso M.J. Formulation of L-asparaginase-loaded poly(lactide-co-glycolide) nanoparticles: influence of polymer properties on enzyme loading, activity and in vitro release. J. Control. Release. 1998;52:53–62. - PubMed
-
- Gref R., Minamitake Y., Peracchia M.T., Trubetskoy V., Torchilin V., Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

