TSLP receptor is not essential for house dust mite-induced allergic rhinitis in mice
- PMID: 28955898
- PMCID: PMC5613305
- DOI: 10.1016/j.bbrep.2016.06.003
TSLP receptor is not essential for house dust mite-induced allergic rhinitis in mice
Abstract
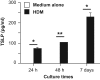
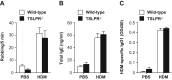
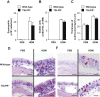
TSLP induces Th2 cytokine production by Th2 cells and various other types of cells, thereby contributing to Th2-type immune responses and development of allergic disorders. We found that house dust mite (HDM) extract induced TSLP production by nasal epithelial cells, suggesting that TSLP may be involved in development of HDM-induced allergic rhinitis (AR). To investigate that possibility in greater detail, wild-type and TSLP receptor-deficient (TSLPR-/-) mice on the C57BL/6J background were repeatedly treated intranasally with HDM extract. The frequency of sneezing, numbers of eosinophils and goblet cells, thickness of submucosal layers, serum levels of total IgE and HDM-specific IgG1, and levels of IL-4, IL-5 and IL-13 in the culture supernatants of HDM-stimulated LN cells were comparable in the two mouse strains. Those findings indicate that, in mice, TSLPR is not crucial for development of HDM-induced AR.
Keywords: AR, allergic rhinitis; Allergy; HDM, house dust mites; House dust mite; Mouse; Rhinitis; TSLP receptor; TSLP, thymic stromal lymphopoietin; TSLPR, thymic stromal lymphopoietin receptor.
Figures

Similar articles
-
Murine allergic rhinitis and nasal Th2 activation are mediated via TSLP- and IL-33-signaling pathways.Int Immunol. 2016 Feb;28(2):65-76. doi: 10.1093/intimm/dxv055. Epub 2015 Oct 1. Int Immunol. 2016. PMID: 26428949 Free PMC article.
-
The influence of house dust mite sublingual immunotherapy on the TSLP-OX40L signaling pathway in patients with allergic rhinitis.Int Forum Allergy Rhinol. 2016 Aug;6(8):862-70. doi: 10.1002/alr.21743. Epub 2016 Mar 25. Int Forum Allergy Rhinol. 2016. PMID: 27012942 Clinical Trial.
-
IL-33, but not IL-25, is crucial for the development of house dust mite antigen-induced allergic rhinitis.PLoS One. 2013 Oct 25;8(10):e78099. doi: 10.1371/journal.pone.0078099. eCollection 2013. PLoS One. 2013. PMID: 24205109 Free PMC article.
-
The effect of immunotherapy on cross-reactivity between house dust mite and other allergens in house dust mite -sensitized patients with allergic rhinitis.Expert Rev Clin Immunol. 2021 Sep;17(9):969-975. doi: 10.1080/1744666X.2021.1968834. Epub 2021 Aug 18. Expert Rev Clin Immunol. 2021. PMID: 34388949 Review.
-
Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate-Adaptive Immunity of Pediatric Asthma.Int J Mol Sci. 2018 Apr 18;19(4):1231. doi: 10.3390/ijms19041231. Int J Mol Sci. 2018. PMID: 29670037 Free PMC article. Review.
Cited by
-
Effectiveness of Indonesian house dust mite allergenic extract in triggering allergic rhinitis sensitivity in a mouse model: A preliminary study.Vet World. 2022 Sep;15(9):2333-2341. doi: 10.14202/vetworld.2022.2333-2341. Epub 2022 Sep 29. Vet World. 2022. PMID: 36341054 Free PMC article.
-
Modulating Th2 Cell Immunity for the Treatment of Asthma.Front Immunol. 2021 Feb 10;12:637948. doi: 10.3389/fimmu.2021.637948. eCollection 2021. Front Immunol. 2021. PMID: 33643321 Free PMC article. Review.
-
Orchestration of epithelial-derived cytokines and innate immune cells in allergic airway inflammation.Cytokine Growth Factor Rev. 2018 Feb;39:19-25. doi: 10.1016/j.cytogfr.2017.11.004. Epub 2017 Nov 21. Cytokine Growth Factor Rev. 2018. PMID: 29169815 Free PMC article. Review.
References
-
- van Cauwenberge P., Bachert C., Passalacqua G., Bousquet J., Canonica G.W., Durham S.R., Fokkens W.J., Howarth P.H., Lund V., Malling H.J., Mygind N., Passali D., Scadding G.K., Wang D.Y. Consensus statement on the treatment of allergic rhinitis. Eur. Acad. Allergol. Clin. Immunol. Allergy. 2000;55:116–134. - PubMed
-
- Skoner D.P. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J. Allergy Clin. Immunol. 2001;108:S2–S8. - PubMed
-
- Zhao C.Q., Li T.L., He S.H., Chen X., An Y.F., Wu W.K., Zhou X.H., Li P., Yang P.C. Specific immunotherapy suppresses Th2 responses via modulating TIM1/TIM4 interaction on dendritic cells. Allergy. 2010;65:986–995. - PubMed
-
- Liu Y.J., Soumelis V., Watanabe N., Ito T., Wang Y.H., Malefyt Rde W., Omori M., Zhou B., Ziegler S.F. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007;25:193–219. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

