Biophysical characterization of the interaction of human albumin with an anionic porphyrin
- PMID: 28955918
- PMCID: PMC5613655
- DOI: 10.1016/j.bbrep.2016.07.014
Biophysical characterization of the interaction of human albumin with an anionic porphyrin
Abstract
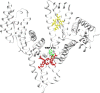
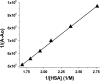
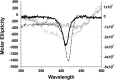
The manuscript describes the characterization of the interaction between meso-tetrakis(p-sulfonatophenyl)porphyrin (TSPP) and human serum albumin (HSA). TSPP is a candidate for the photosensitization of structural and functional changes in proteins while HSA provides both an excellent protein model and binding and functional characteristics that could be explored in future applications of the approach. A combination of optical spectroscopic techniques (e.g., fluorescence spectroscopy, fluorescence lifetime, circular dichroism, etc.) and computational docking simulations were applied to better characterize the TSPP/HSA interaction. Recent advances have revealed that the complex formed by TSPP and HSA has become potentially relevant to biomedical applications, biomaterials research and protein photosensitized engineering. The study has determined a likely location of the binding site that places TSPP at a site that overlaps partially with the low affinity site of ibuprofen and places one of the [Formula: see text] groups of the ligand in proximity of the Trp214 residue in HSA. The characterization will enable future studies aimed at photosensitizing non-native functions of HSA for biomedical and biomaterial applications.
Keywords: Albumin; Docking; Porphyrin; Spectroscopy.
Figures



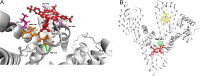
Similar articles
-
Multi-Spectroscopic and Molecular Modeling Studies of Interactions Between Anionic Porphyrin and Human Serum Albumin.Int J Mol Sci. 2024 Nov 20;25(22):12473. doi: 10.3390/ijms252212473. Int J Mol Sci. 2024. PMID: 39596539 Free PMC article.
-
Spectroscopic studies on the interaction of a water soluble porphyrin and two drug carrier proteins.Biophys J. 2002 Mar;82(3):1607-19. doi: 10.1016/S0006-3495(02)75512-4. Biophys J. 2002. PMID: 11867473 Free PMC article.
-
Femtosecond to second studies of a water-soluble porphyrin derivative in chemical and biological nanocavities.Langmuir. 2012 Mar 6;28(9):4363-72. doi: 10.1021/la204949e. Epub 2012 Feb 24. Langmuir. 2012. PMID: 22324339
-
[The Interaction Between a New Water-Soluble Meso-Tetrakis(Carboxyl) Zinc(Ⅱ) Porphyrin and Human Serum Albumin].Guang Pu Xue Yu Guang Pu Fen Xi. 2016 Sep;36(9):2894-2900. Guang Pu Xue Yu Guang Pu Fen Xi. 2016. PMID: 30084622 Chinese.
-
Binding of porphyrins to tubulin heterodimers.Biomacromolecules. 2007 Dec;8(12):3767-78. doi: 10.1021/bm700687x. Epub 2007 Nov 19. Biomacromolecules. 2007. PMID: 18020394
Cited by
-
Systemic Effects of Photoactivated 5,10,15,20-tetrakis(N-methylpyridinium-3-yl) Porphyrin on Healthy Drosophila melanogaster.BioTech (Basel). 2024 Jul 3;13(3):23. doi: 10.3390/biotech13030023. BioTech (Basel). 2024. PMID: 39051338 Free PMC article.
-
Novel combinations of experimental and computational analysis tested on the binding of metalloprotoporphyrins to albumin.Int J Biol Macromol. 2019 Aug 1;134:445-457. doi: 10.1016/j.ijbiomac.2019.05.060. Epub 2019 May 10. Int J Biol Macromol. 2019. PMID: 31078597 Free PMC article.
-
Multi-wavelength analytical ultracentrifugation of human serum albumin complexed with porphyrin.Eur Biophys J. 2018 Oct;47(7):789-797. doi: 10.1007/s00249-018-1301-7. Epub 2018 Apr 19. Eur Biophys J. 2018. PMID: 29675648 Free PMC article.
-
Nonlinear van't Hoff Behavior in the Interaction of Two Water-Soluble Porphyrins with Bovine Serum Albumin (BSA).ACS Omega. 2024 Nov 20;9(48):47699-47709. doi: 10.1021/acsomega.4c07367. eCollection 2024 Dec 3. ACS Omega. 2024. PMID: 39651067 Free PMC article.
-
Dendrimer porphyrins as the oxygen sensor for intracellular imaging to suppress interaction towards biological molecules.J Clin Biochem Nutr. 2019 Nov;65(3):178-184. doi: 10.3164/jcbn.19-13. Epub 2019 Sep 27. J Clin Biochem Nutr. 2019. PMID: 31777418 Free PMC article.
References
-
- Guallar V., Wallrapp F.H. QM/MM methods: Looking inside heme proteins biochemistry. Biophys. Chem. 2010;149:1–11. - PubMed
-
- Kennedy J.C., Marcus S.L., Pottier R.H. Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): mechanisms and clinical results. J. Clin. Las. Med. Surg. 1996;14:289–304. - PubMed
-
- Komatsu T., Wang R.-M., Zunszain P.A., Curry S., Tsuchida E. Photosensitized reduction of water to hydrogen using human serum albumin complexed with zinc-protoporphyin IX. J. AM Chem. Soc. 2006;128:16297–16301. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

