Activation of conventional and novel protein kinase C isozymes by different diacylglycerol molecular species
- PMID: 28955926
- PMCID: PMC5613651
- DOI: 10.1016/j.bbrep.2016.07.017
Activation of conventional and novel protein kinase C isozymes by different diacylglycerol molecular species
Abstract
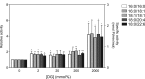
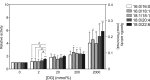
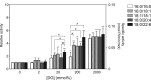
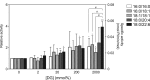
A variety of diacylglycerol (DG) molecular species are produced in stimulated cells. Conventional (α, βII and γ) and novel (δ, ε, η and θ) protein kinase C (PKC) isoforms are known to be activated by DG. However, a comprehensive analysis has not been performed. In this study, we analyzed activation of the PKC isozymes in the presence of 2-2000 mmol% 16:0/16:0-, 16:0/18:1-, 18:1/18:1-, 18:0/20:4- or 18:0/22:6-DG species. PKCα activity was strongly increased by DG and exhibited less of a preference for 18:0/22:6-DG at 2 mmol%. PKCβII activity was moderately increased by DG and did not have significant preference for DG species. PKCγ activity was moderately increased by DG and exhibited a moderate preference for 18:0/22:6-DG at 2 mmol%. PKCδ activity was moderately increased by DG and exhibited a preference for 18:0/22:6-DG at 20 and 200 mmol%. PKCε activity moderately increased by DG and showed a moderate preference for 18:0/22:6-DG at 2000 mmol%. PKCη was not markedly activated by DG. PKCθ activity was the most strongly increased by DG and exhibited a preference for 18:0/22:6-DG at 2 and 20 mmol% DG. These results indicate that conventional and novel PKCs have different sensitivities and dependences on DG and a distinct preference for shorter and saturated fatty acid-containing and longer and polyunsaturated fatty acid-containing DG species, respectively. This differential regulation would be important for their physiological functions.
Keywords: Activation; DG, diacylglycerol; DGK, diacylglycerol kinase; Diacylglycerol; Isoform; MBP, myelin basic protein fragment 4-14; Molecular species; PKC, protein kinase C; Protein kinase C; TPA, 12-O-Tetradecanoylphorbol-13-acetate; cPKC, conventional protein kinase C; nPKC, novel protein kinase C.
Figures




References
-
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. - PubMed
-
- Ron D., Kazanietz M.G. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999;13:1658–1676. - PubMed
-
- Zeng L., Webster S.V., Newton P.M. The biology of protein kinase C. Adv. Exp. Med. Biol. 2012;740:639–661. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

