Monosialoganglioside-GM1 triggers binding of the amyloid-protein salmon calcitonin to a Langmuir membrane model mimicking the occurrence of lipid-rafts
- PMID: 28955978
- PMCID: PMC5614544
- DOI: 10.1016/j.bbrep.2016.10.005
Monosialoganglioside-GM1 triggers binding of the amyloid-protein salmon calcitonin to a Langmuir membrane model mimicking the occurrence of lipid-rafts
Abstract
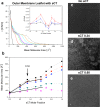
GM1 ganglioside is known to be involved in the amyloid-associated diseases and it is a crucial factor for the assembly of amyloid proteins on lipid-rafts, which are lipid structures located on the synaptic plasma membranes. Due to its slow aggregation rate, we employed salmon calcitonin (sCT) as a suitable probe representative of amyloid proteins, to study the interaction between this class of proteins and a membrane model. Here, we prepared a neuronal membrane model by depositing onto mica two Langmuir-Blodgett films in liquid-condensed phase: the outer monolayer was characterized by high content of GM1 (50%) and minority parts of cholesterol and POPC (25-25%), while the inner one by plain POPC. To deeply investigate the interaction of sCT with this model and the role-played by GM1, we prepared the outer leaflet adding sCT at a concentration such that the number of proteins equals that of GM1. Atomic Force Microscopy revealed the occurrence of two distinct kinds of flat surfaces, with globular aggregates localized exclusively on top of the highest one. To unravel the nature of the interaction, we studied by ζ-potential technique liposomes composed as the outer leaflet of the model. Results demonstrated that an electrostatic interaction sCT-GM1 occurred. Finally, to investigate the interaction thermodynamics between sCT and the outer leaflet, Langmuir films as the outer monolayer and containing increasing content of sCT were studied by compression isotherms and Brewster Angle Microscopy experiments. Based on the all body of results we propose an interaction model where GM1 plays a pivotal role.
Keywords: AFM; Amyloid; BAM; GM1; Langmuir Films; Lipid-rafts; Membrane Models; sCT; ζ-potential.
Figures






Similar articles
-
The Interaction between Amyloid Prefibrillar Oligomers of Salmon Calcitonin and a Lipid-Raft Model: Molecular Mechanisms Leading to Membrane Damage, Ca2+-Influx and Neurotoxicity.Biomolecules. 2019 Dec 29;10(1):58. doi: 10.3390/biom10010058. Biomolecules. 2019. PMID: 31905804 Free PMC article.
-
Surface chemistry of lipid raft and amyloid Aβ (1-40) Langmuir monolayer.Colloids Surf B Biointerfaces. 2011 Oct 15;87(2):369-77. doi: 10.1016/j.colsurfb.2011.05.047. Epub 2011 Jun 12. Colloids Surf B Biointerfaces. 2011. PMID: 21708455
-
Calcitonin forms oligomeric pore-like structures in lipid membranes.Biophys J. 2006 Sep 15;91(6):2275-81. doi: 10.1529/biophysj.105.079475. Biophys J. 2006. PMID: 16940475 Free PMC article.
-
Atomic force microscopy studies of a floating-bilayer lipid membrane on a Au(111) surface modified with a hydrophilic monolayer.Langmuir. 2011 Sep 6;27(17):10867-77. doi: 10.1021/la2016269. Epub 2011 Jul 29. Langmuir. 2011. PMID: 21766864
-
Role of Electrostatic Interactions in Calcitonin Prefibrillar Oligomer-Induced Amyloid Neurotoxicity and Protective Effect of Neuraminidase.Int J Mol Sci. 2021 Apr 11;22(8):3947. doi: 10.3390/ijms22083947. Int J Mol Sci. 2021. PMID: 33920464 Free PMC article.
Cited by
-
Designing a Useful Lipid Raft Model Membrane for Electrochemical and Surface Analytical Studies.Molecules. 2021 Sep 9;26(18):5483. doi: 10.3390/molecules26185483. Molecules. 2021. PMID: 34576954 Free PMC article.
-
Amyloid Prefibrillar Oligomers: The Surprising Commonalities in Their Structure and Activity.Int J Mol Sci. 2021 Jun 16;22(12):6435. doi: 10.3390/ijms22126435. Int J Mol Sci. 2021. PMID: 34208561 Free PMC article. Review.
-
Molecular mechanisms at the basis of the protective effect exerted by EPPS on neurodegeneration induced by prefibrillar amyloid oligomers.Sci Rep. 2024 Nov 3;14(1):26533. doi: 10.1038/s41598-024-77859-9. Sci Rep. 2024. PMID: 39489758 Free PMC article.
-
Calcitonin native prefibrillar oligomers but not monomers induce membrane damage that triggers NMDA-mediated Ca2+-influx, LTP impairment and neurotoxicity.Sci Rep. 2019 Mar 26;9(1):5144. doi: 10.1038/s41598-019-41462-0. Sci Rep. 2019. PMID: 30914688 Free PMC article.
-
Characterization and Fluctuations of an Ivermectin Binding Site at the Lipid Raft Interface of the N-Terminal Domain (NTD) of the Spike Protein of SARS-CoV-2 Variants.Viruses. 2024 Nov 27;16(12):1836. doi: 10.3390/v16121836. Viruses. 2024. PMID: 39772146 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

