Vibrational spectroscopy and imaging: applications for tissue engineering
- PMID: 28956032
- PMCID: PMC5653442
- DOI: 10.1039/c7an01055a
Vibrational spectroscopy and imaging: applications for tissue engineering
Abstract
Tissue engineering (TE) approaches strive to regenerate or replace an organ or tissue. The successful development and subsequent integration of a TE construct is contingent on a series of in vitro and in vivo events that result in an optimal construct for implantation. Current widely used methods for evaluation of constructs are incapable of providing an accurate compositional assessment without destruction of the construct. In this review, we discuss the contributions of vibrational spectroscopic assessment for evaluation of tissue engineered construct composition, both during development and post-implantation. Fourier transform infrared (FTIR) spectroscopy in the mid and near-infrared range, as well as Raman spectroscopy, are intrinsically label free, can be non-destructive, and provide specific information on the chemical composition of tissues. Overall, we examine the contribution that vibrational spectroscopy via fiber optics and imaging have to tissue engineering approaches.
Figures
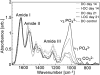
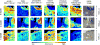
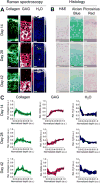
References
-
- Langer R, Vacanti JP. Science. 1993;260:920–926. - PubMed
-
- U.-U. N. f. O. Sharing. UNOS annual report. https://www.unos.org/about/annual-report/, (accessed June 14 2017, 2017)
-
- Song LJ, Murphy SV, Yang B, Xu YM, Zhang YY, Atala A. Tissue Engineering Part B-Reviews. 2014;20:163–172. - PubMed
-
- Kawecki M, Łabuś W, Klama-Baryla A, Kitala D, Kraut M, Glik J, Misiuga M, Nowak M, Bielecki T, Kasperczyk A. J Biomed Mater Res B Appl Biomater. 2017 - PubMed
-
- Consolo F, Brizzola S, Tremolada G, Grieco V, Riva F, Acocella F, Fiore GB, Soncini M. Journal of Tissue Engineering and Regenerative Medicine. 2016;10:E101–E112. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

