Clinical Utility of Expanded Carrier Screening: Reproductive Behaviors of At-Risk Couples
- PMID: 28956228
- PMCID: PMC5943379
- DOI: 10.1007/s10897-017-0160-1
Clinical Utility of Expanded Carrier Screening: Reproductive Behaviors of At-Risk Couples
Abstract
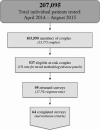
Expanded carrier screening (ECS) analyzes dozens or hundreds of recessive genes to determine reproductive risk. Data on the clinical utility of screening conditions beyond professional guidelines are scarce. Individuals underwent ECS for up to 110 genes. Five-hundred thirty-seven at-risk couples (ARC), those in which both partners carry the same recessive disease, were invited to participate in a retrospective IRB-approved survey of their reproductive decision making after receiving ECS results. Sixty-four eligible ARC completed the survey. Of 45 respondents screened preconceptionally, 62% (n = 28) planned IVF with PGD or prenatal diagnosis (PNDx) in future pregnancies. Twenty-nine percent (n = 13) were not planning to alter reproductive decisions. The remaining 9% (n = 4) of responses were unclear. Of 19 pregnant respondents, 42% (n = 8) elected PNDx, 11% (n = 2) planned amniocentesis but miscarried, and 47% (n = 9) considered the condition insufficiently severe to warrant invasive testing. Of the 8 pregnancies that underwent PNDx, 5 were unaffected and 3 were affected. Two of 3 affected pregnancies were terminated. Disease severity was found to have significant association (p = 0.000145) with changes in decision making, whereas guideline status of diseases, controlled for severity, was not (p = 0.284). Most ARC altered reproductive planning, demonstrating the clinical utility of ECS. Severity of conditions factored into decision making.
Keywords: Clinical utility; Expanded carrier screening; Preimplantation genetic diagnosis; Prenatal diagnosis.
Conflict of interest statement
Conflict of Interest
Caroline Ghiossi received a stipend for a student internship and has consulted for Counsyl Inc.
Kenny K. Wong, James D. Goldberg, and Gabriel A. Lazarin are employees of Counsyl Inc.
Imran S. Haque is a former employee of Counsyl Inc.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human Studies and Informed Consent
Informed consent was obtained from all individual participants included in the study.
Animal Studies
This article does not contain any studies with animals performed by any of the authors.
Figures
References
-
- Committee Opinion No. 690 (2017). Obstetrics & Gynecology, 129(3). doi:10.1097/aog.0000000000001951. - PubMed
-
- Exome Aggregation Consortium (ExAC), (2016) Cambridge, MA (URL: http://exac.broadinstitute.org/variant/3-15686693-G-C) [June 2016].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous