A method for volumetric retinal tissue oxygen tension imaging
- PMID: 28956656
- PMCID: PMC6037488
- DOI: 10.1080/02713683.2017.1373823
A method for volumetric retinal tissue oxygen tension imaging
Abstract
Purpose: Inadequate retinal oxygenation occurs in many vision-threatening retinal diseases, including diabetic retinopathy, retinal vascular occlusions, and age-related macular degeneration. Therefore, techniques that assess retinal oxygenation are necessary to understand retinal physiology in health and disease. The purpose of the current study is to report a method for the three-dimensional (3D) imaging of retinal tissue oxygen tension (tPO2) in rats.
Methods: Imaging was performed in Long Evans pigmented rats under systemic normoxia (N = 6) or hypoxia (N = 3). A vertical laser line was horizontally scanned on the retina and a series of optical section phase-delayed phosphorescence images were acquired. From these images, phosphorescence volumes at each phase delay were constructed and a 3D retinal tPO2 volume was generated. Retinal tPO2 volumes were quantitatively analyzed by generating retinal depth profiles of mean tPO2 (MtPO2) and the spatial variation of tPO2 (SVtPO2). The effects of systemic condition (normoxia/hypoxia) and retinal depth on MtPO2 and SVtPO2 were determined by mixed linear model.
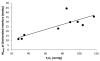
Results: Each 3D retinal tPO2 volume was approximately 500 × 750 × 200 μm (horizontal × vertical × depth) and consisted of 45 en face tPO2 images through the retinal depth. MtPO2 at the chorioretinal interface was significantly correlated with systemic arterial oxygen tension (P = 0.007; N = 9). There were significant effects of both systemic condition and retinal depth on MtPO2 and SVtPO2, such that both were lower under hypoxia than normoxia and higher in the outer retina than inner retina (P < 0.001).
Conclusion: For the first time, 3D imaging of retinal tPO2 was demonstrated, with potential future application for assessment of physiological alterations in animal models of retinal diseases.
Keywords: Rat; phosphorescence lifetime imaging; retina; three-dimensional imaging; tissue oxygen tension.
Conflict of interest statement
None (AEF, JW, PT, NPB); patent (MS).
Mahnaz Shahidi holds a patent for the imaging technology. None of the authors have received financial support or have personal financial interest relevant to the topic of the manuscript.
Figures
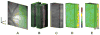
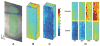
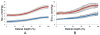
Similar articles
-
Quantifying retinal oxygenation and metabolism by phosphorescence lifetime imaging.Exp Eye Res. 2025 Aug;257:110422. doi: 10.1016/j.exer.2025.110422. Epub 2025 May 15. Exp Eye Res. 2025. PMID: 40381978 Review.
-
Retinal tissue oxygen tension and consumption during light flicker stimulation in rat.Exp Eye Res. 2018 Oct;175:207-211. doi: 10.1016/j.exer.2018.08.007. Epub 2018 Aug 16. Exp Eye Res. 2018. PMID: 30121195 Free PMC article.
-
A Method for Combined Retinal Vascular and Tissue Oxygen Tension Imaging.Sci Rep. 2017 Sep 6;7(1):10622. doi: 10.1038/s41598-017-10955-1. Sci Rep. 2017. PMID: 28878307 Free PMC article.
-
Response of inner retinal oxygen extraction fraction to light flicker under normoxia and hypoxia in rat.Invest Ophthalmol Vis Sci. 2014 Sep 2;55(9):6055-8. doi: 10.1167/iovs.13-13811. Invest Ophthalmol Vis Sci. 2014. PMID: 25183761 Free PMC article.
-
Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease.Prog Retin Eye Res. 2001 Mar;20(2):175-208. doi: 10.1016/s1350-9462(00)00027-6. Prog Retin Eye Res. 2001. PMID: 11173251 Review.
Cited by
-
Retinal Oxygen Delivery, Metabolism, and Extraction Fraction during Long-Term Bilateral Common Carotid Artery Occlusion in Rats.Sci Rep. 2020 Jun 25;10(1):10371. doi: 10.1038/s41598-020-67255-4. Sci Rep. 2020. PMID: 32587289 Free PMC article.
-
In vivo O2 imaging in hepatic tissues by phosphorescence lifetime imaging microscopy using Ir(III) complexes as intracellular probes.Sci Rep. 2020 Dec 3;10(1):21053. doi: 10.1038/s41598-020-76878-6. Sci Rep. 2020. PMID: 33273499 Free PMC article.
-
Quantifying retinal oxygenation and metabolism by phosphorescence lifetime imaging.Exp Eye Res. 2025 Aug;257:110422. doi: 10.1016/j.exer.2025.110422. Epub 2025 May 15. Exp Eye Res. 2025. PMID: 40381978 Review.
-
Retinal tissue oxygen tension and consumption during light flicker stimulation in rat.Exp Eye Res. 2018 Oct;175:207-211. doi: 10.1016/j.exer.2018.08.007. Epub 2018 Aug 16. Exp Eye Res. 2018. PMID: 30121195 Free PMC article.
-
Two-photon phosphorescence lifetime microscopy of retinal capillary plexus oxygenation in mice.J Biomed Opt. 2018 Dec;23(12):1-9. doi: 10.1117/1.JBO.23.12.126501. J Biomed Opt. 2018. PMID: 30516039 Free PMC article.
References
-
- Kiel JW. The ocular circulation. Morgan & Claypool Life Sciences; San Rafael, CA, USA: 2010. http://www.ncbi.nlm.nih.gov/books/NBK53329/ - PubMed
-
- Dartt DA, Besharse JC, Dana R. Encyclopedia of the eye. Elsevier, Academic Press; Cambridge, MA, USA: 2010.
-
- Cringle SJ, Yu DY, Yu PK, Su EN. Intraretinal oxygen consumption in the rat in vivo. Invest Ophthalmol Vis Sci. 2002;43(6):1922–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
