Genesis and Spread of Newly Emerged Highly Pathogenic H7N9 Avian Viruses in Mainland China
- PMID: 28956760
- PMCID: PMC5686710
- DOI: 10.1128/JVI.01277-17
Genesis and Spread of Newly Emerged Highly Pathogenic H7N9 Avian Viruses in Mainland China
Abstract
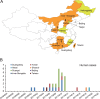
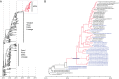
The novel low-pathogenic avian influenza A H7N9 viruses (LPAI H7N9 viruses) have been a threat to public health since their emergence in 2013 because of the high rates of mortality and morbidity that they cause. Recently, highly pathogenic variants of these avian influenza A H7N9 viruses (HPAI H7N9 viruses) have emerged and caused human infections and outbreaks among poultry in mainland China. However, it is still unclear how the HPAI H7N9 virus was generated and how it evolved and spread in China. Here, we show that the ancestor virus of the HPAI H7N9 viruses originated in the Yangtze River Delta region and spread southward to the Pearl River Delta region, possibly through live poultry trade. After introduction into the Pearl River Delta region, the origin LPAI H7N9 virus acquired four amino acid insertions in the hemagglutinin (HA) protein cleavage site and mutated into the HPAI H7N9 virus in late May 2016. Afterward, the HPAI H7N9 viruses further reassorted with LPAI H7N9 or H9N2 viruses locally and generated multiple different genotypes. As of 14 July 2017, the HPAI H7N9 viruses had spread from Guangdong Province to at least 12 other provinces. The rapid geographical expansion and genetic evolution of the HPAI H7N9 viruses pose a great challenge not only to public health but also to poultry production. Effective control measures, including enhanced surveillance, are therefore urgently needed.IMPORTANCE The LPAI H7N9 virus has caused five outbreak waves in humans and was recently reported to have mutated into highly pathogenic variants. It is unknown how the HPAI H7N9 virus originated, evolved, and disseminated in China. In this study, we comprehensively analyzed the sequences of HPAI H7N9 viruses from 28 human and 21 environmental samples covering eight provinces in China that were taken from November 2016 to June 2017. The results show that the ancestor virus of the HPAI H7N9 viruses originated in the Yangtze River Delta region. However, the insertion of four amino acids into the HA protein cleavage site of an LPAI H7N9 virus occurred in late May 2016 in the Pearl River Delta region. The mutated HPAI H7N9 virus further reassorted with LPAI H7N9 or H9N2 viruses that were cocirculating in poultry. Considering the rapid geographical expansion of the HPAI H7N9 viruses, effective control measures are urgently needed.
Keywords: genesis; geographic dissemination; highly pathogenic H7N9 avian viruses; origins.
Copyright © 2017 American Society for Microbiology.
Figures



References
-
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368:1888–1897. doi:10.1056/NEJMoa1304459. - DOI - PubMed
-
- Wang X, Jiang H, Wu P, Uyeki TM, Feng L, Lai S, Wang L, Huo X, Xu K, Chen E, Wang X, He J, Kang M, Zhang R, Zhang J, Wu J, Hu S, Zhang H, Liu X, Fu W, Ou J, Wu S, Qin Y, Zhang Z, Shi Y, Zhang J, Artois J, Fang VJ, Zhu H, Guan Y, Gilbert M, Horby PW, Leung GM, Gao GF, Cowling BJ, Yu H. 2017. Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013-17: an epidemiological study of laboratory-confirmed case series. Lancet Infect Dis 17:822–832. doi:10.1016/S1473-3099(17)30323-7. - DOI - PMC - PubMed
-
- Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, Kong H, Gu C, Li X, Liu J, Wang G, Chen Y, Liu L, Liang L, Li Y, Fan J, Wang J, Li W, Guan L, Li Q, Yang H, Chen P, Jiang L, Guan Y, Xin X, Jiang Y, Tian G, Wang X, Qiao C, Li C, Bu Z, Chen H. 2013. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 341:410–414. doi:10.1126/science.1240532. - DOI - PubMed
-
- World Health Organization. 2017. Human infection with avian influenza A(H7N9) virus—China. Disease outbreak news. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/don/27-february-2017-ah7n9-china/en/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

