Differentiated Human SH-SY5Y Cells Provide a Reductionist Model of Herpes Simplex Virus 1 Neurotropism
- PMID: 28956768
- PMCID: PMC5686721
- DOI: 10.1128/JVI.00958-17
Differentiated Human SH-SY5Y Cells Provide a Reductionist Model of Herpes Simplex Virus 1 Neurotropism
Abstract
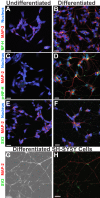
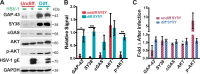
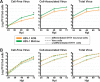
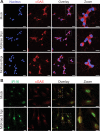
Neuron-virus interactions that occur during herpes simplex virus (HSV) infection are not fully understood. Neurons are the site of lifelong latency and are a crucial target for long-term suppressive therapy or viral clearance. A reproducible neuronal model of human origin would facilitate studies of HSV and other neurotropic viruses. Current neuronal models in the herpesvirus field vary widely and have caveats, including incomplete differentiation, nonhuman origins, or the use of dividing cells that have neuropotential but lack neuronal morphology. In this study, we used a robust approach to differentiate human SH-SY5Y neuroblastoma cells over 2.5 weeks, producing a uniform population of mature human neuronal cells. We demonstrate that terminally differentiated SH-SY5Y cells have neuronal morphology and express proteins with subcellular localization indicative of mature neurons. These neuronal cells are able to support a productive HSV-1 infection, with kinetics and overall titers similar to those seen in undifferentiated SH-SY5Y cells and the related SK-N-SH cell line. However, terminally differentiated, neuronal SH-SY5Y cells release significantly less extracellular HSV-1 by 24 h postinfection (hpi), suggesting a unique neuronal response to viral infection. With this model, we are able to distinguish differences in neuronal spread between two strains of HSV-1. We also show expression of the antiviral protein cyclic GMP-AMP synthase (cGAS) in neuronal SH-SY5Y cells, which is the first demonstration of the presence of this protein in nonepithelial cells. These data provide a model for studying neuron-virus interactions at the single-cell level as well as via bulk biochemistry and will be advantageous for the study of neurotropic viruses in vitroIMPORTANCE Herpes simplex virus (HSV) affects millions of people worldwide, causing painful oral and genital lesions, in addition to a multitude of more severe symptoms such as eye disease, neonatal infection, and, in rare cases, encephalitis. Presently, there is no cure available to treat those infected or prevent future transmission. Due to the ability of HSV to cause a persistent, lifelong infection in the peripheral nervous system, the virus remains within the host for life. To better understand the basis of virus-neuron interactions that allow HSV to persist within the host peripheral nervous system, improved neuronal models are required. Here we describe a cost-effective and scalable human neuronal model system that can be used to study many neurotropic viruses, such as HSV, Zika virus, dengue virus, and rabies virus.
Keywords: HSV-1; SH-SY5Y; SK-N-SH; cGAS; differentiation; herpes simplex virus; infection; neuron; virus.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
In vitro susceptibility of differentiated SH-SY5Y human neuroblastoma cells to herpes simplex virus type 1 and Japanese encephalitis virus infection.J Vet Med Sci. 2025 Apr 1;87(4):426-430. doi: 10.1292/jvms.24-0405. Epub 2025 Feb 24. J Vet Med Sci. 2025. PMID: 39993732 Free PMC article.
-
Lund Human Mesencephalic (LUHMES) Neuronal Cell Line Supports Herpes Simplex Virus 1 Latency In Vitro.J Virol. 2019 Mar 5;93(6):e02210-18. doi: 10.1128/JVI.02210-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30602607 Free PMC article.
-
Contemporary Circulating Enterovirus D68 Strains Have Acquired the Capacity for Viral Entry and Replication in Human Neuronal Cells.mBio. 2018 Oct 16;9(5):e01954-18. doi: 10.1128/mBio.01954-18. mBio. 2018. PMID: 30327438 Free PMC article.
-
Herpes Simplex Virus 1 Infection of Neuronal and Non-Neuronal Cells Elicits Specific Innate Immune Responses and Immune Evasion Mechanisms.Front Immunol. 2021 May 31;12:644664. doi: 10.3389/fimmu.2021.644664. eCollection 2021. Front Immunol. 2021. PMID: 34135889 Free PMC article. Review.
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
Cited by
-
Comprehensive analysis of differential expression profiles via transcriptome sequencing in SH-SY5Y cells infected with CV-A16.PLoS One. 2020 Nov 6;15(11):e0241174. doi: 10.1371/journal.pone.0241174. eCollection 2020. PLoS One. 2020. PMID: 33156879 Free PMC article.
-
In vitro susceptibility of differentiated SH-SY5Y human neuroblastoma cells to herpes simplex virus type 1 and Japanese encephalitis virus infection.J Vet Med Sci. 2025 Apr 1;87(4):426-430. doi: 10.1292/jvms.24-0405. Epub 2025 Feb 24. J Vet Med Sci. 2025. PMID: 39993732 Free PMC article.
-
Modeling Varicella Zoster Virus Persistence and Reactivation - Closer to Resolving a Perplexing Persistent State.Front Microbiol. 2019 Jul 24;10:1634. doi: 10.3389/fmicb.2019.01634. eCollection 2019. Front Microbiol. 2019. PMID: 31396173 Free PMC article. Review.
-
Differential Tick Salivary Protein Profiles and Human Immune Responses to Lone Star Ticks (Amblyomma americanum) From the Wild vs. a Laboratory Colony.Front Immunol. 2019 Aug 28;10:1996. doi: 10.3389/fimmu.2019.01996. eCollection 2019. Front Immunol. 2019. PMID: 31555263 Free PMC article.
-
Ochratoxin A induces global DNA hypomethylation and oxidative stress in neuronal cells in vitro.Mycotoxin Res. 2020 Feb;36(1):73-81. doi: 10.1007/s12550-019-00370-y. Epub 2019 Aug 22. Mycotoxin Res. 2020. PMID: 31441013
References
-
- Roizman B, Knipe DM, Whitley R. 2013. Herpes simplex viruses, p 1823–1897. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

