BARHL1 Is Downregulated in Alzheimer's Disease and May Regulate Cognitive Functions through ESR1 and Multiple Pathways
- PMID: 28956815
- PMCID: PMC5664095
- DOI: 10.3390/genes8100245
BARHL1 Is Downregulated in Alzheimer's Disease and May Regulate Cognitive Functions through ESR1 and Multiple Pathways
Abstract
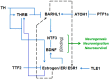
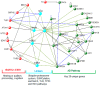
The Transcription factor BarH like homeobox 1 (BARHL1) is overexpressed in medulloblastoma and plays a role in neurogenesis. However, much about the BARHL1 regulatory networks and their functions in neurodegenerative and neoplastic disorders is not yet known. In this study, using a tissue microarray (TMA), we report for the first time that BARHL1 is downregulated in hormone-negative breast cancers and Alzheimer's disease (AD). Furthermore, using an integrative bioinformatics approach and mining knockout mouse data, we show that: (i) BARHL1 and Estrogen Receptor 1 (ESR1) may constitute a network that regulates Neurotrophin 3 (NTF3)- and Brain Derived Neurotrophic Factor (BDNF)-mediated neurogenesis and neural survival; (ii) this is probably linked to AD pathways affecting aberrant post-translational modifications including SUMOylation and ubiquitination; (iii) the BARHL1-ESR1 network possibly regulates β-amyloid metabolism and memory; and (iv) hsa-mir-18a, having common key targets in the BARHL1-ESR1 network and AD pathway, may modulate neuron death, reduce β-amyloid processing and might also be involved in hearing and cognitive decline associated with AD. We have also hypothesized why estrogen replacement therapy improves AD condition. In addition, we have provided a feasible new mechanism to explain the abnormal function of mossy fibers and cerebellar granule cells related to memory and cognitive decline in AD apart from the Tau and amyloid pathogenesis through our BARHL1-ESR1 axis.
Keywords: Alzheimer’s disease; bioinformatics; estrogen; microRNA; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Barhl1 regulates migration and survival of cerebellar granule cells by controlling expression of the neurotrophin-3 gene.J Neurosci. 2004 Mar 24;24(12):3104-14. doi: 10.1523/JNEUROSCI.4444-03.2004. J Neurosci. 2004. PMID: 15044550 Free PMC article.
-
BARHL1 homeogene, the human ortholog of the mouse Barhl1 involved in cerebellum development, shows regional and cellular specificities in restricted domains of developing human central nervous system.Biochem Biophys Res Commun. 2006 Jan 6;339(1):296-304. doi: 10.1016/j.bbrc.2005.11.021. Epub 2005 Nov 15. Biochem Biophys Res Commun. 2006. PMID: 16307728
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Disease modifying effect of chronic oral treatment with a neurotrophic peptidergic compound in a triple transgenic mouse model of Alzheimer's disease.Neurobiol Dis. 2014 Nov;71:110-30. doi: 10.1016/j.nbd.2014.07.001. Epub 2014 Jul 15. Neurobiol Dis. 2014. PMID: 25046994
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
A Plasma Circular RNA Profile Differentiates Subjects with Alzheimer's Disease and Mild Cognitive Impairment from Healthy Controls.Int J Mol Sci. 2022 Oct 31;23(21):13232. doi: 10.3390/ijms232113232. Int J Mol Sci. 2022. PMID: 36362022 Free PMC article.
-
Neurotrophin-3 Promotes the Neuronal Differentiation of BMSCs and Improves Cognitive Function in a Rat Model of Alzheimer's Disease.Front Cell Neurosci. 2021 Feb 10;15:629356. doi: 10.3389/fncel.2021.629356. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33642999 Free PMC article.
-
Precision Behavioral Management (PBM) and Cognitive Control as a Potential Therapeutic and Prophylactic Modality for Reward Deficiency Syndrome (RDS): Is There Enough Evidence?Int J Environ Res Public Health. 2022 May 24;19(11):6395. doi: 10.3390/ijerph19116395. Int J Environ Res Public Health. 2022. PMID: 35681980 Free PMC article. Review.
-
Integrated network-based multiple computational analyses for identification of co-expressed candidate genes associated with neurological manifestations of COVID-19.Sci Rep. 2022 Oct 13;12(1):17141. doi: 10.1038/s41598-022-21109-3. Sci Rep. 2022. PMID: 36229517 Free PMC article.
-
Study on the Mechanism of Acori Graminei Rhizoma in the Treatment of Alzheimer's Disease Based on Network Pharmacology and Molecular Docking.Biomed Res Int. 2021 Dec 24;2021:5418142. doi: 10.1155/2021/5418142. eCollection 2021. Biomed Res Int. 2021. PMID: 34977242 Free PMC article.
References
-
- Alzheimer’s Association 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–168. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

