Role of the Aryl Hydrocarbon Receptor in Sugen 5416-induced Experimental Pulmonary Hypertension
- PMID: 28956952
- PMCID: PMC5854960
- DOI: 10.1165/rcmb.2017-0260OC
Role of the Aryl Hydrocarbon Receptor in Sugen 5416-induced Experimental Pulmonary Hypertension
Abstract
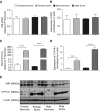
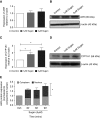
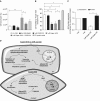
Rats dosed with the vascular endothelial growth factor inhibitor Sugen 5416 (Su), subjected to hypoxia, and then restored to normoxia have become a widely used model of pulmonary arterial hypertension (PAH). However, the mechanism by which Su exacerbates pulmonary hypertension is unclear. We investigated Su activation of the aryl hydrocarbon receptor (AhR) in human pulmonary artery smooth muscle cells (hPASMCs) and blood outgrowth endothelial cells (BOECs) from female patients with PAH. We also examined the effect of AhR on aromatase and estrogen levels in the lung. Protein and mRNA analyses demonstrated that CYP1A1 was very highly induced in the lungs of Su/hypoxic (Su/Hx) rats. The AhR antagonist CH223191 (8 mg/kg/day) reversed the development of PAH in this model in vivo and normalized lung CYP1A1 expression. Increased lung aromatase and estrogen levels in Su/Hx rats were also normalized by CH223191, as was AhR nuclear translocator (ARNT [HIF-1β]), which is shared by HIF-1α and AhR. Su reduced HIF-1α expression in hPASMCs. Su induced proliferation in BOECs and increased apoptosis in human pulmonary microvascular ECs and also induced translocation of AhR to the nucleus in hPASMCs. Under normoxic conditions, hPASMCs did not proliferate to Su. However, when grown in hypoxia (1%), Su induced hPASMC proliferation. In combination with hypoxia, Su is proliferative in hPASMCs and BOECs from patients with PAH, and Su/Hx-induced PAH in rats may be facilitated by AhR-induced CYP1A1, ARNT, and aromatase. Inhibition of AhR may be a novel approach to the treatment of pulmonary hypertension.
Keywords: Sugen; VEGF; aryl hydrocarbon receptor; estrogen; pulmonary hypertension.
Figures



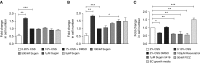
References
-
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12) Suppl S:13S–24S. - PubMed
-
- Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, McMahon G, Waltenberger J, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. - PubMed
-
- Mendel DB, Schreck RE, West DC, Li G, Strawn LM, Tanciongco SS, et al. The angiogenesis inhibitor SU5416 has long-lasting effects on vascular endothelial growth factor receptor phosphorylation and function. Clin Cancer Res. 2000;6:4848–4858. - PubMed
-
- Sukbuntherng J, Cropp G, Hannah A, Wagner GS, Shawver LK, Antonian L. Pharmacokinetics and interspecies scaling of a novel VEGF receptor inhibitor, SU5416. J Pharm Pharmacol. 2001;53:1629–1636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

